Professor of Chemistry
nagorny@umich.eduOffice Information:
3807 Chemistry
phone: 7346152833
Catalysis; Organic Chemistry; Pharmaceutical Chemistry; Chemistry; Synthesis; Photochemistry
Education/Degree:
B.S. Oregon State University, Ph.D., Harvard University, PostDoc, Memorial Sloan-Kettering Cancer CenterAbout
Research in my group is focused on the areas of organic synthesis and catalysis with the long-term goals of (1) developing and exploring new catalytic transformations with an emphasis on organocatalytic transformations, and (2) developing concise syntheses of bioactive natural products (NPs) and using these syntheses as a platform for designing new therapeutic agents for the treatment of human diseases. These goals represent highly synergistic areas. Access to new catalytic transformations could significantly enhance the preparation and evaluation of bioactive NPs, which is of great importance to the field of drug discovery as more than 50% of approved therapeutic agents are derived from or mimic NPs. At the same time, the synthesis of bioactive NPs helps to identify the shortcomings of the existing synthetic methods and drives the development of new catalytic reactions. The students in my group are trained in various areas of organic synthesis and catalysis as well as in the areas of medicinal and computational chemistry.
Focus Area 1. Developing Concise Asymmetric Syntheses of Natural Products. Bioactive natural products have served as an essential and continuous source of therapeutic agents for the treatment of various human diseases. The ability to synthetically generate bioactive natural products and their derivatives has been of great importance to the field of drug discovery. Despite some great advances in the synthesis of certain classes of natural products, many important types of medicinally valuable natural products and their derivatives are still not readily available by synthesis. We have identified cardiotonic steroids, bioactive diterpenes, and glutarimide-based macrolactones as important families of bioactive natural products, biological studies which would greatly benefit from having simple and concise syntheses of these molecules. A primary goal for our group in these studies is to develop general, scalable, and modular approaches to the aforementioned classes of natural products and edit the structure of these compounds to improve their biological properties. Some of our recent progress in this area is summarized in the publications below (publications 2, 6, 9, 10, and 16).
Focus Area 2. Selective Chiral Catalyst-Controlled Functionalization of Natural Products. One of our major research programs is focused on discovering new catalytic methods directed to simplify the chemical functionalization of complex natural products using small molecule-based chiral catalysts rather than enzymes. In our recently published efforts, we have explored the use of chiral Brønsted acids as simple organic catalysts mimicking the function of glycosyltransferases. Our group has developed chiral phosphoric acid-based catalysts that can promote highly site-selective acetalization of monosaccharide-derived polyols. Building on these results, we have achieved chiral Brønsted acid-controlled site-selective glycosylations of 14-membered macrolide-derived triols and tetraols and regioselective desymmetrizative glycosylation of 6-deoxystreptamine derivatives. In our collaborative efforts, we have also demonstrated that these reactions proceed through the covalently linked glycosyl phosphates, which also provides an opportunity to design various stoichiometric solid-supported reagents and catalysts for site-selective glycosylation. These methodologies may provide direct access to glycosylated derivatives of complex natural polyols thus significantly simplifying the preparation of such derivatives. In addition to glycosylation, other types of functionalizations are currently being explored using organic Bronsted acids as catalysts.
Focus Area 3. Exploring Organocatalytic Transformations of Oxocarbenium Ions. The development of stereoselective and regioselective transformations of organic molecules represents one of the fundamental challenges in modern organic synthesis, and a lack of selective methods may significantly affect the availability and purity of biologically active NPs and therapeutic agents. Among such gaps is the lack of catalyst-controlled transformations involving oxocarbenium ions. The chemistry of oxocarbenium ions is central to the preparation of many medicinally important classes of compounds such as glycosides, acetals, spiroketals as well as oxygen-containing heterocyclic compounds. The therapeutic properties of these compounds strongly depend on the stereochemical features and position of the acetal linkage within the molecule. However, synthetic installation of these features is particularly challenging for the reactions involving oxocarbenium ions. This part of our research program focuses on identifying new techniques to control the selective formation of acetals by harnessing the reactivity of oxocarbenium ions using chiral catalysts. Some of our recent progress in this area is summarized in the publications below.
Focus Area 4. Asymmetric catalysis, chiral ligand and catalyst design, and new synthetic method development. The design and development of new chiral ligands and catalysts have been one of the important directions in our group. These studies are highly synergistic with the other research directions described above as asymmetric catalysis has played the central role in the majority of the synthetic and methodological studies in which our group has been involved. Based on our experiences in spiroketalization studies, our group has designed a new class of spiroketal-based chiral scaffolds termed SPIROLs. The SPIROL-based ligands have demonstrated excellent performance in a variety of Pd-, Rh- and Ir-catalyzed transformations while being significantly more accessible than other types of spirocyclic ligands. Our group continues further development and investigation of the SPIROL-based ligands, in particular, in the context of the cardiotonic steroid and catalyst-controlled natural product functionalization studies. This includes the exploration of SPIROL-based ligands for the enantioselective Robinson-annulation leading to quaternary stereocenter-containing polycyclic molecules recently reported in our group as well as site-selective diastereoselective functionalization of natural products.
In addition, our group has contributed to improving the methods available for the preparation and characterization of the existing chiral scaffolds and catalysts as well as designing new recyclable chiral Brønsted acid-based organocatalysts that are immobilized on the polymer matrix. We are also interested in designing new methods and strategies that would improve access to important building blocks and functionalities.
Submitted for review and preprints:
A) Krishnarjuna, B.; Sharma, G.; Hiiuk, V.; Struppe, J.; Nagorny, P.; Ivanova, M.; Ramamoorthy, A. "Nanodisc reconstitution and characterization of amyloid-b-precursor protein C99" 2023, Submitted. Access PrePrint Here.
Published Work:
60) Tichvon, C.; Zviagin, E.; Surma, Z.; Nagorny, P.* "Concise Synthesis of Bufadienolide Cinobufagin via Late-Stage Singlet Oxygen Oxidation/Rearrangement Approach" Org. Lett. 2024, 26, 2445.
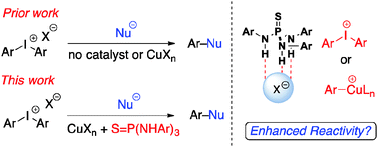
59) Zhelavskyi, O.; Parikh, S.; Staples, R. J.; Zimmerman, P. M.,* Nagorny, P.* "Green Light Promoted Iridium(III)/Copper(I)-Catalyzed Addition of Alkynes to Aziridinoquinoxalines Through the Intermediacy of Azomethine Ylides" Angew. Chem. Int. Ed. 2024, e202318876, DOI: 10.1002/anie.202318876
Access the ChemRxiv PrePrint here.
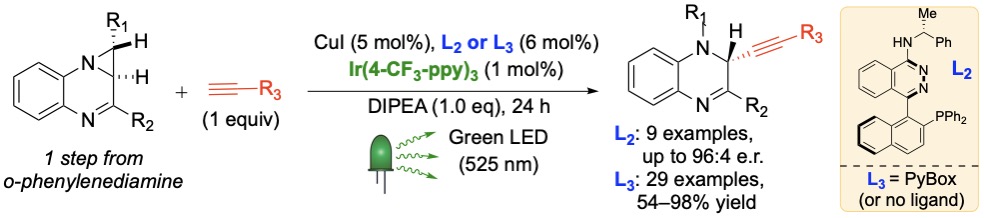
58) Rutkoski, R.; Debarba, L. K.; Stilgenbauer, L.; Rosenthal, T.; Sadagurski, M.;* Nagorny, P.* "Selective (a)-L-Rhamnosylation and Neuroprotective Activity Exploration of Cardiotonic Steroids" ACS Med. Chem. Lett. 2024, 15, 280.
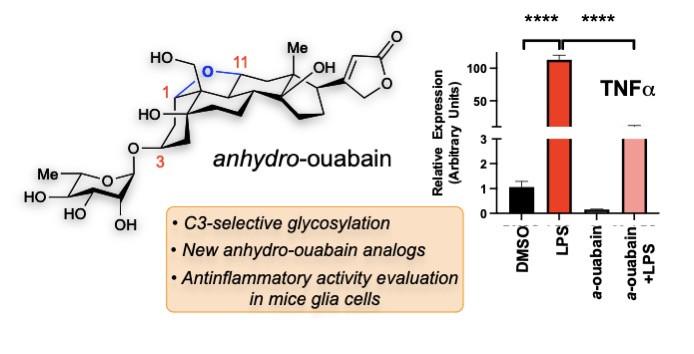
57. Rutkoski, R.; Arguelles, A. J.; Huang, Q.; Nagorny, P.* "Development of Recyclable Polystyrene Supported Phosphonic Acid Resins for Carbohydrate Immobilization and Glycosylation" J. Org. Chem. 2023, 88, 16467. Access Article Here.
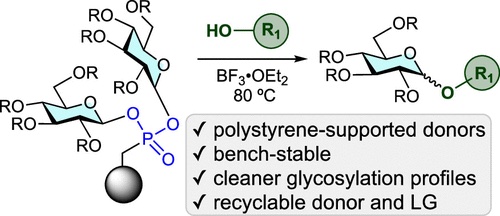
56. Jhang, Y.-J.#; Zhelavskyi, O.#; Nagorny, P.*; “Enantioselective Parallel Kinetic Resolution of Aziridine-Containing Quinoxalines via Chiral Phosphoric Acid-Catalyzed Transfer Hydrogenation”Org.Lett. 2023, 25, 7721. Access Article Here
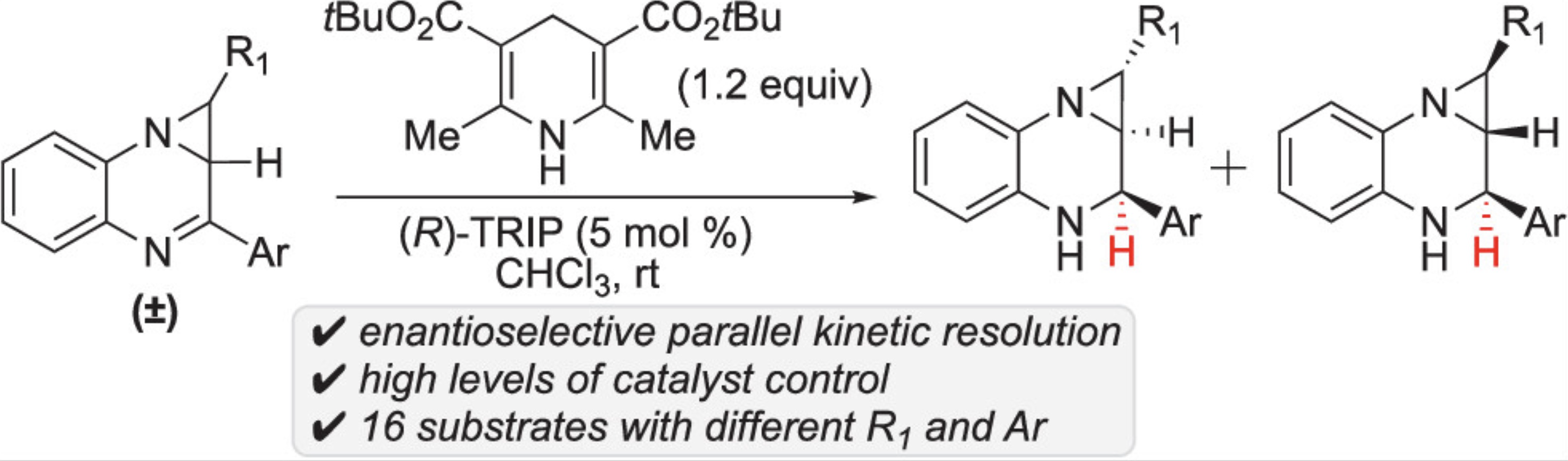
55. Zhelavskyi, O.; Jhang, Y.-J.; Nagorny, P.*; “Asymmetric Transfer Hydrogenation of Heterocyclic Compounds in Continuous Flow Using an Immobilized Chiral Phosphoric Acid as the Catalyst” Synthesis 2023, 55, 2361 (Special Edition Dedicated to D. A. Evans). Access Article Here
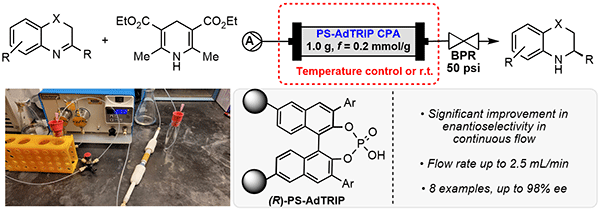
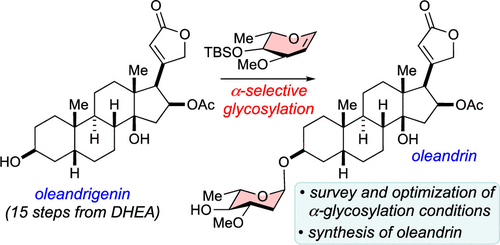
54. Carney, N.; Perry, N.; Garabedian, J.; Nagorny, P. "Development of a-Selective Glycosylation with L-Oleandral and Its Application to the Total Synthesis of Oleandrin" Org. Lett. 2023, 6, 966. Access Article Here
53. Eid, S.; Zerbes, T.; Williams, D.; Wang, X. Z.; Sackmann, C.; Meier, S.; Dulin, N. O.; Nagorny, P. Schmitt-Ulms, G. “Indentification of a Cardiac Glycoside Exhibiting Favorable Brain Bioavailability and Potency for Reducing Levels of the Cellular Prion Protein” Int. J. Mol. Sci. 2022, 23, 14823. Access Article Here.
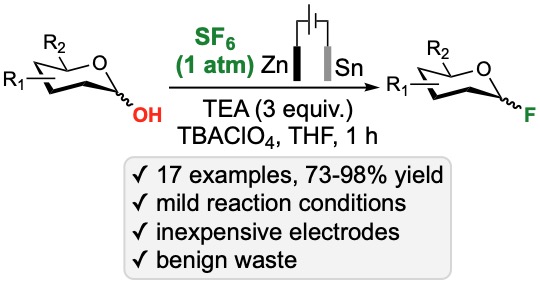
52. Kim, S.; Nagorny, P. "Electrochemical Fluorination with Sulfur(VI) Hexafluoride for the Synthesis of Glycosyl Fluoride" Org. Lett. 2022, 24, 2294. Access Article Here.
51. Diaz, N.; Nagorny, P. "(11bR)-4-Hydroxy-2,6-bis[2,4,6-tris(1-methylethyl)phenyl]-dinaphtho[2,1-d: 1',2'-f][1,3,2]dioxaphosphepin-4-oxide, [(R)-TRIP] & (11bS)-4-Hydroxy-2,6-bis[2,4,6-tris(1-methylethyl)phenyl]-dinaphtho[2,1-d: 1',2'-f][1,3,2]dioxaphosphepin-4-oxide, [(S)-TRIP]" (Update) Encyclopedia of Reagents in Organic Synthesis 2022. Access Article Here.
50. Sun, S.; Fejedelem, Z.; Song, S.; Nagorny, P. "Synthesis of (((1R,3S,3'S)-3,3'-diethyl-3H,3'H-1,1'-spirobi[isobenzofuran]-7,7'-diyl)bis(oxy))bis(diphenylphosphane) Org. Syn. 2022, 99, 190.
49. Sun, S.; Nagorny, P. "Synthesis of Chiral Aziridine Ligands for Asymmetric Alkylation with Alkylzincs: diphenyl((S)-1-((S)-1-phenylethyl)aziridin-2-yl)methanol" Org. Syn. 2021, 98, 446. Access Article Here.
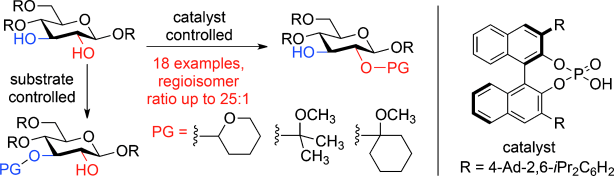
48. Wang, S.; Zhelavskyi, O.; Lee, J.; Arguelles, A. J.; Khomutnyk, Y.; Mensah, E. A.; Guo, H.; Hourani, R.; Zimmerman, P. M.; Nagorny, P. "Studies of catalyst-controlled regioselective acetalization and its application to single-pot synthesis of differentially protected saccharides" J. Am. Chem. Soc. 2021, 143, 18592. Access Article Here
Pre Print: ChemRxiv. PrePrint. Access Article Here.
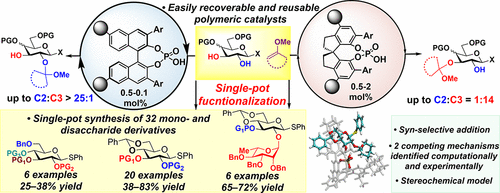
47. Sun, S.; Nagorny, P. " Synthesis and Evaluation of C2-Symmetric SPIROL-based bis-Oxazoline Ligands" Symmetry 2021, 13, 1667. (Special Edition dedicated to Symmetric and Asymmetric Total Synthesis). Access Article Here.
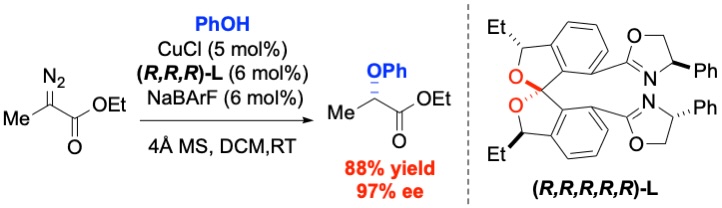
46. Fejedelem, Z.; Carney, N.; Nagorny, P. "Synthesis of cardiotonic steroids oleandrigenin and rhodexin B" J. Org. Chem. 2021, 86, 10249. Access Article Here.
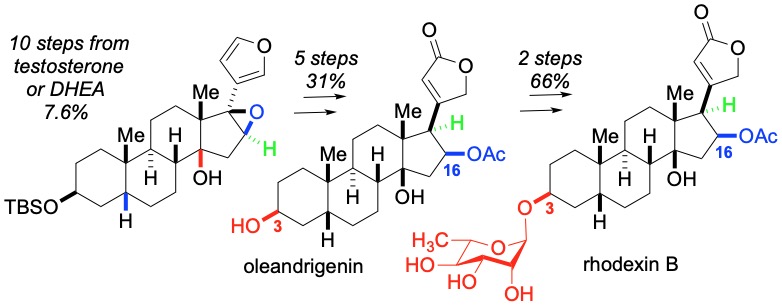
45. Kim, S.; Khomutnyk, Y.; Bannykh, A.; Nagorny, P. "Synthesis of glycosyl fluorides by photochemical fluorination with sulfur(VI) hexafluoride" Org. Lett. 2021, 23, 190. (ACS Editor's Choice). Access this manuscript here.
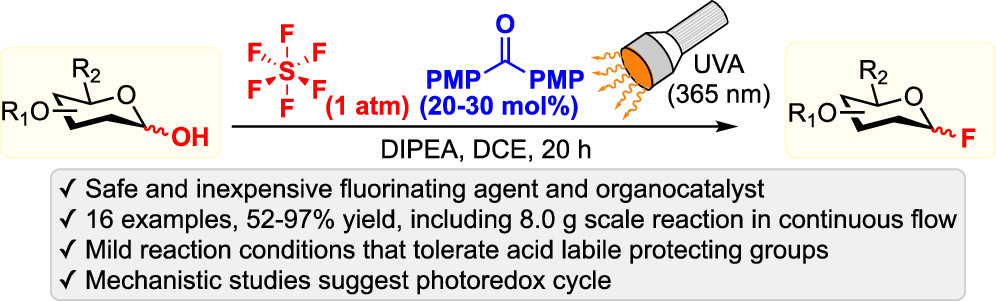
44. Sun, S.; Nagorny, P. "Exploration of chiral diastereomeric spiroketal (SPIROL)-based phosphinite ligands in asymmetric hydrogenation of heterocycles" Chem. Commun. 2020, 56, 8432.
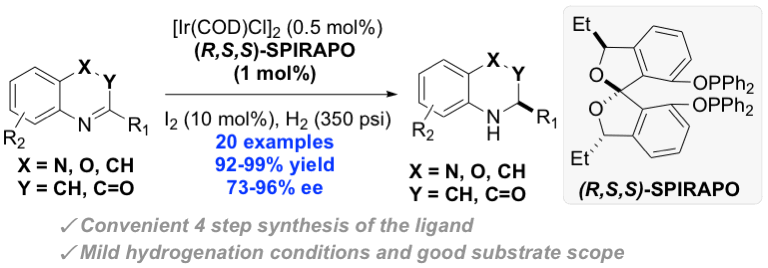
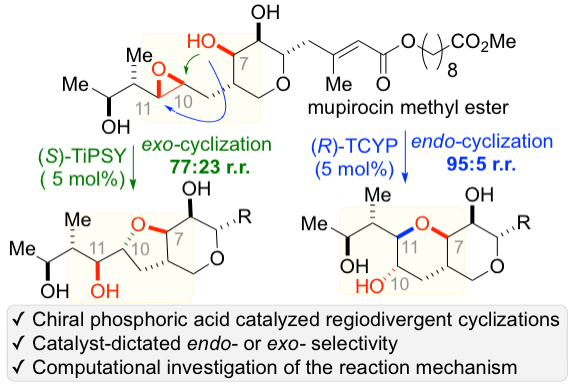
43. Wang, S.; Arguelles, A. J.; Tay, J.-H.; Hotta, M.; Zimmerman, P. M.*; Nagorny, P.* "Experimental and computational studies on regiodivergent chiral phosphoric acid catalyzed cycloisomerization of mupirocin methyl ester" Chem. Eur. J. 2020, 26, 4583 (Hot Article). Access Paper Here.
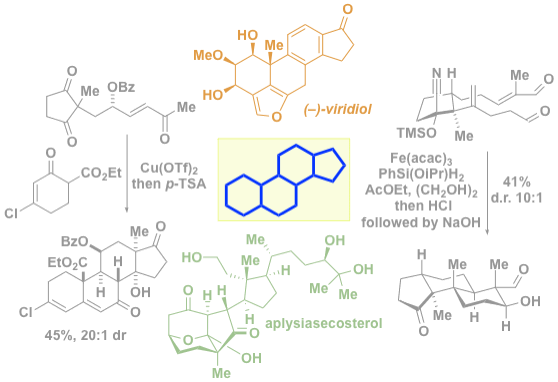
42. Khatri, H. R.; Carney, N.; Rutkoski, R.; Bhattarai, B.; Nagorny, P. "Recent progress in steroid synthesis triggered by the emergence of new catalytic methods" Eur. J. Org. Chem. 2020, 21, 755 (Invited Minireview); Access Paper Here.
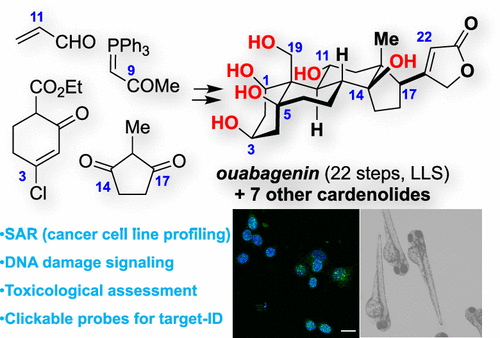
41.Khatri, H. R.; Bhattarai, B.; Kaplan, W.; Li, Z.; Long, M. J. C.;* Aye, Y.;* Nagorny, P.* "Modular total synthesis and cell-based anticancer activity evaluation of ouabagenin and other cardiotonic steroids with varying degrees of oxygenation" J. Am. Chem. Soc. 2019, 141, 4849. Access Paper Here.
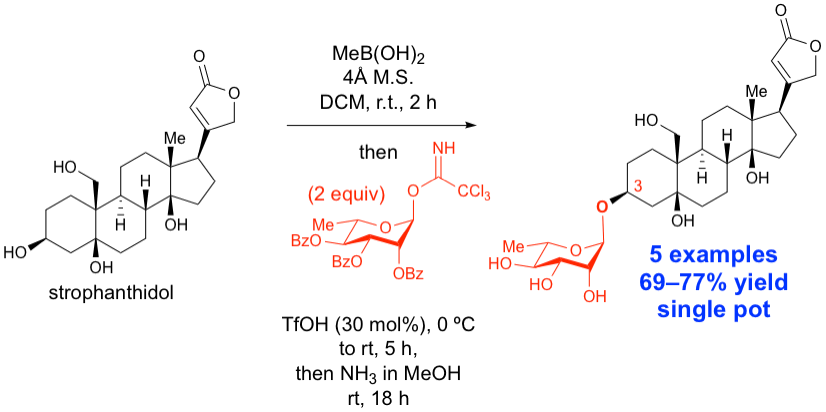
40.Tay, J.-H.; Dorokhov, V.; Wang, S.; Nagorny, P. "Regioselective Single Pot C3-Glycosylation of Strophanthidol Using Methylboronic Acid as a Traceless Protecting Group" J. Antibiotics, 2019, 72, 437 (Invited Contribution to the Special Issue Dedicated to Professor Samuel Danishefsky). Access paper here.
39.Kearney, S. E. et al. "Canvass: A Crowd-Sourced, Natural-Product Screening Library for Exploring Biological Space" ACS Cent. Sci., 2018, 4, 1727. Access paper here.
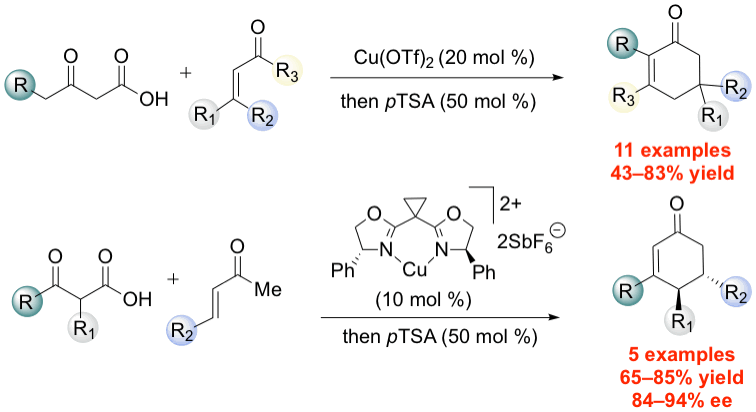
38. Lee, J.; Wang, S.; Callahan, M.; Nagorny, P.* "Copper(II)-catalyzed tandem decarboxylative Michael/Aldol reactions leading to the formation of functionalized cyclohexenones" Org. Lett., 2018, 20, 2067. Access paper here.
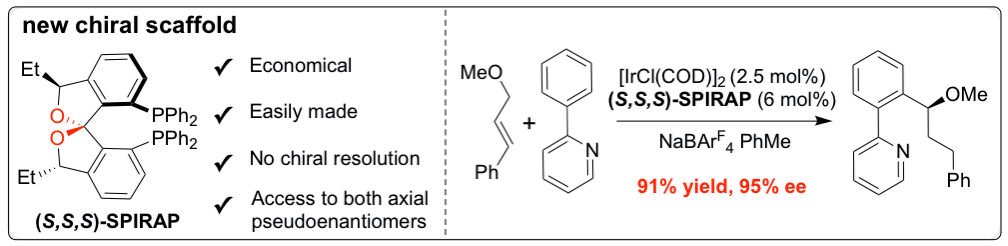
37. Arguelles, A. J.; Sun, S.; Budaitis, B.; Nagorny, P.* "Design, synthesis, and application of chiral C2-symmetric spiroketal-containing ligands in transition-metal catalysis" Angew. Chem. Int. Ed., 2018, 57, 5325. Access paper here.
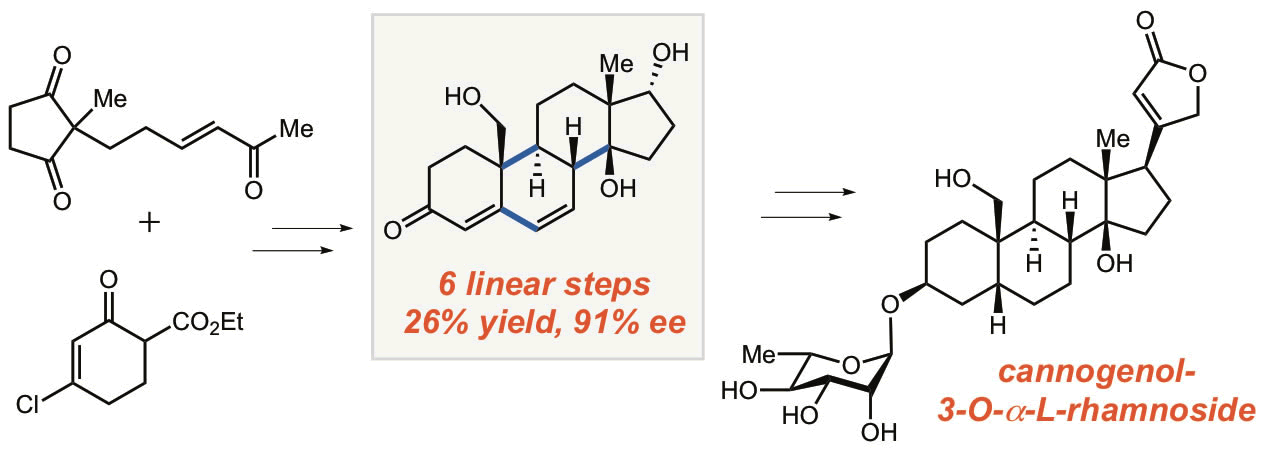
36. Bhattarai, B.; Nagorny, P.* "Enantioselective total synthesis of cannogenol-3-O-a-L-rhamnoside via sequential Cu(II)-catalyzed Michael addition/intramolecular aldol cyclization reactions" Org.Lett., 2018, 20, 154. Access paper here.
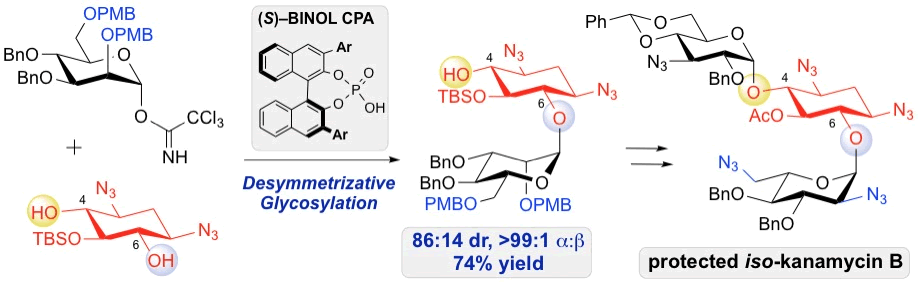
35. Lee, J.; Borovika, A.; Khomutnyk, Y.; Nagorny, P.* "Chiral phosphoric acid-catalyzed desymmetrizative glycosylation of 2-deoxystreptamine and its application to aminoglycoside synthesis" Chem. Commun., 2017, 53, 8976. (themed issue "Site Selective Molecular Transformations"). Access paper here.
34. Tay, J.-H.; Nagorny, P.* "Bronsted Acid Catalysts Derived from Phosphorous" Non-nitregenous Organocatalysis, Chapter 3, 2017 (edited by Andrew Harned), In Production.
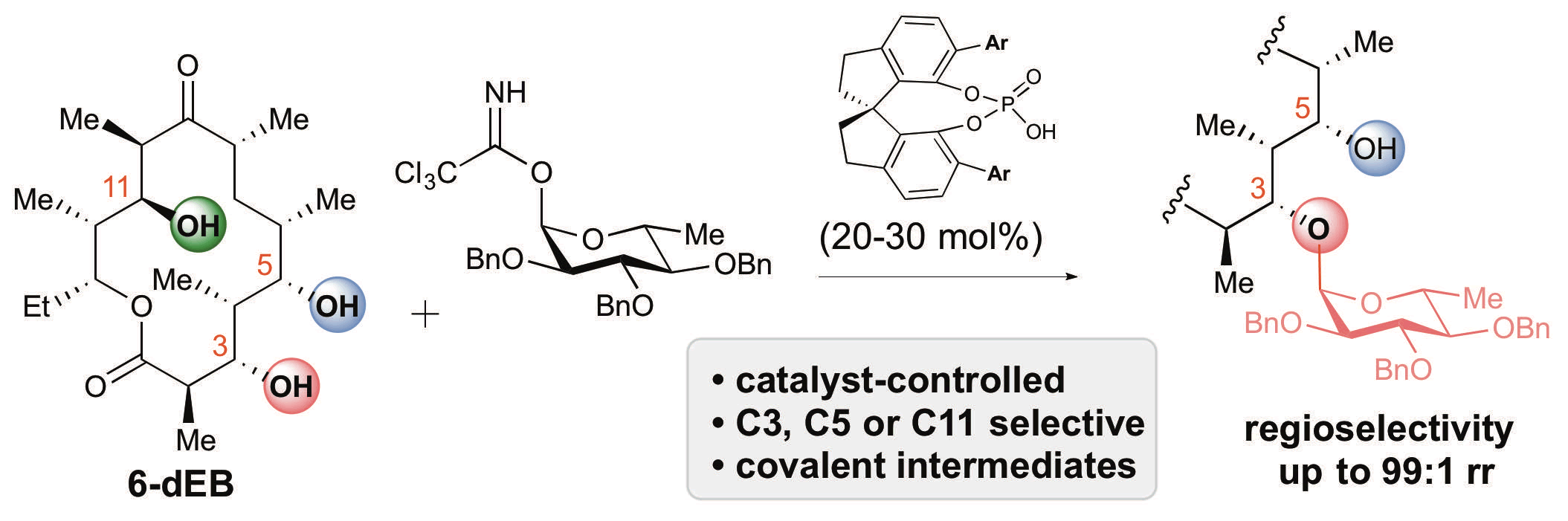
33. Tay, J.-H.; Arguelles, A. J.; DeMars II, M. D.; Zimmerman, P. M.;* Sherman, D. H.;* Nagorny, P.* "Regiodivergent Glycosylations of 6-deoxy-Erythronolide B and Oleandomycin-derivied Macrolides Enabled by Chiral Acid Catalysis" J. Am. Chem. Soc. 2017, 139, 8570. Access paper here.
32. Nagorny, P.;* Sun, Z.* "New Approaches to Organocatalysis Based on C–H and C–X bonding for Electrophilic Substrate Activation" Beilst. J. Org. Chem. 2016, 12, 2834. Access paper here.
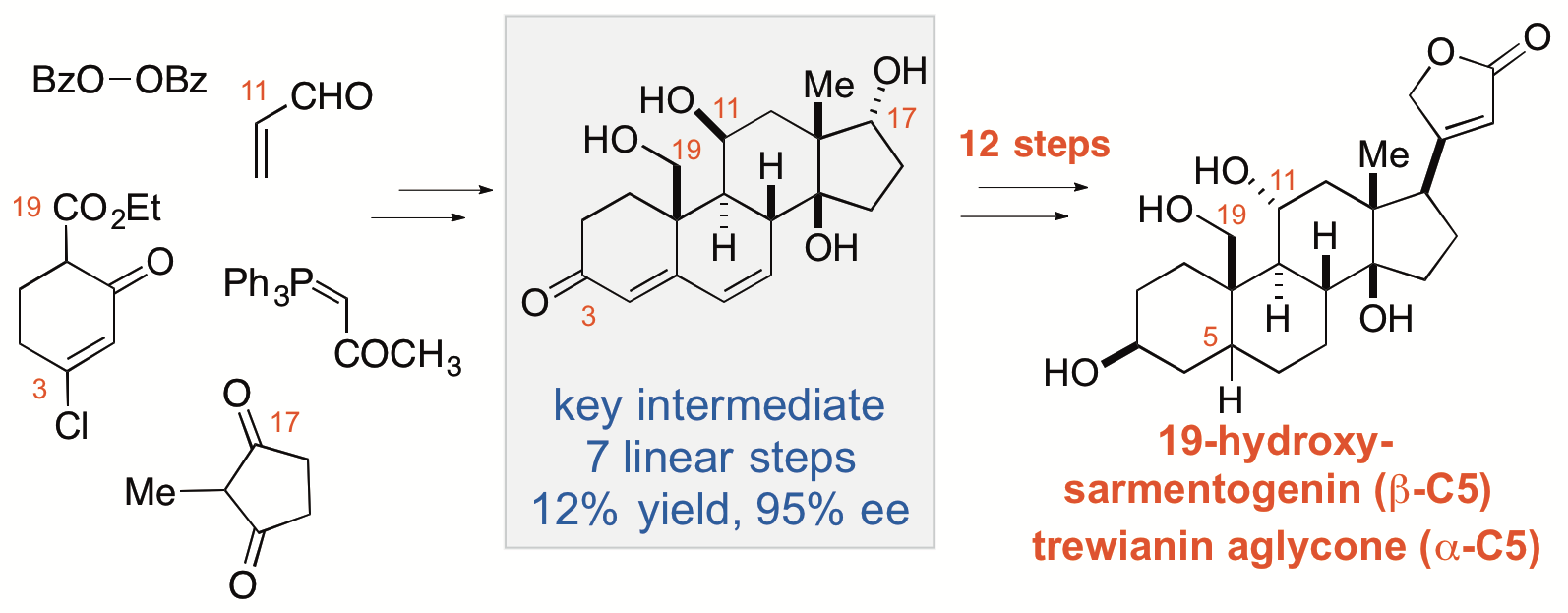
31. Kaplan, W.; Khatri, H. R.; Nagorny, P. "Concise Enantioselective Total Synthesis of Cardiotonic Steroids 19-Hydroxysarmentogenin and Trewianin Aglycone" J. Am. Chem. Soc. 2016, 138, 7194. Access paper here.
30. Nagorny, P.; Cichowicz, N. "New Strategy Based on Sequential Michael/Aldol Reactions for the Asymmetric Synthesis of Cardenolides" Strategies and Tactics in Organic Synthesis 12 (Book chapter), Volume 12, Chapter 9, 2016 (edited by Michael Harmata), 238-266.
29. Khomutnyk, Y. Y.; Arguelles, A. J.; Winschel, G. A.; Sun, Z.; Zimmerman, P. M.;* Nagorny, P.* "Studies of the Mechanism and Origins of Enantioselectivity for the Chiral Phosphoric Acid-Catalyzed Stereoselective Spiroketalization" J. Am. Chem. Soc. 2016, 138, 444.Access paper here
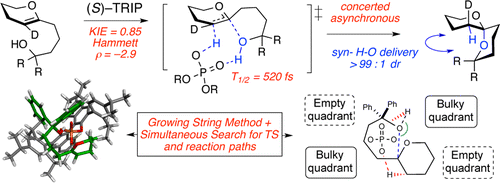
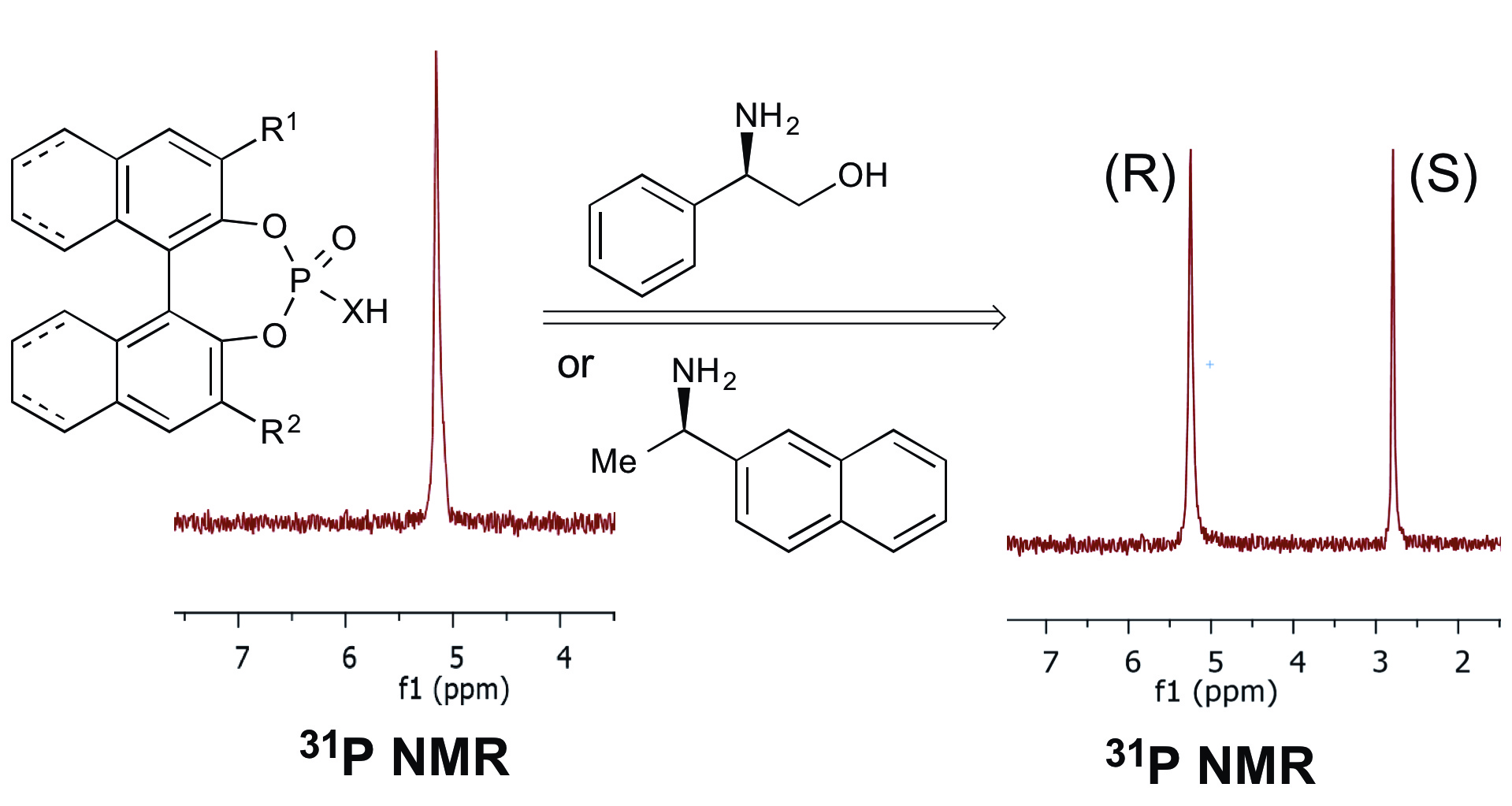
28. Tay, J.-H.; Nagorny, P.* "31P NMR-based Method for Determining Enantiopurity of Chiral Phosphoric Acids, and Its Application to the BINOL- and H8-BINOL-based Chiral Phosphoric Acid Thermal Racemization Studies" Synlett (Invited submission for Asymmetric Phosphoric Acid Catalysis Cluster) 2016, 27(04), 551-554. Access paper here.
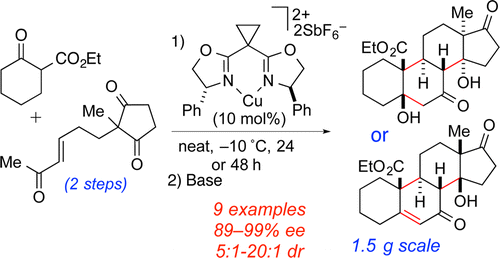
27.Cichowicz, N.; Kaplan, W.; Khomutnik, I.; Bhattarai, B.; Sun, Z.; Nagorny, P.* "Concise enantioselective synthesis of oxygenated steroids via sequential copper(II)-catalyzed Michael addition/intramolecular aldol cyclization reactions" J. Am. Chem. Soc. 2015, 137, 14341. (One of the top 20 most downloaded JACS publications in the month of December). Access paper here.
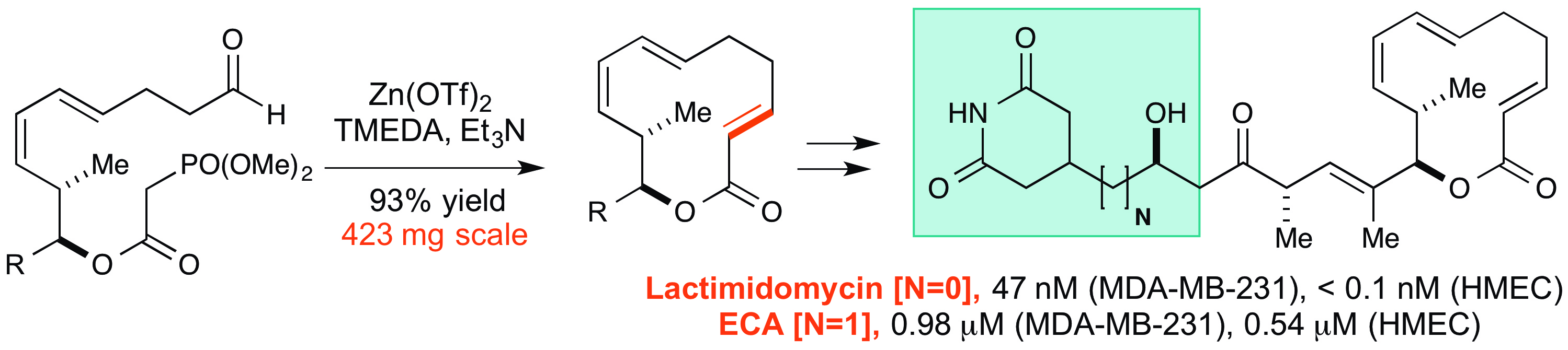
26. Larsen, B. J.; Sun, Z.; Lachacz, E.; Khomutnik, I.; Soellner, M. B.*; Nagorny, P.* "Synthesis and Biological Evaluation of Lactimidomycin and its Analogs" Chem. Eur. J. 2015, 21, 19159.Access paper here.
25. Tay, J.-H.; Arguelles, J. A.; Nagorny, P.* "Direct Interconversion of BINOL and H8-BINOL-based Chiral Bronsted Acids Using Single Step Red/Ox Manipulations" Org. Lett.2015, 17, 3774. Access paper here
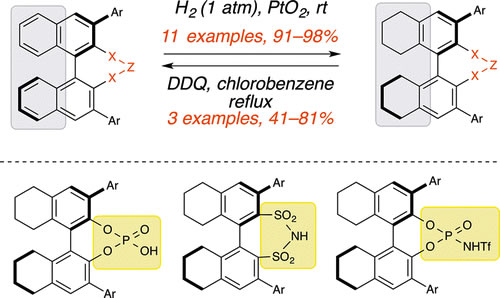
24. Bhattarai, B.; Tay, J.-H.; Nagorny, P.* "Thiophosphoramides as Cooperative Catalysts for Copper-Catalyzed Arylation of Carboxylates with Diaryliodonium Salts" Chem. Commun. 2014, 51, 5398. Access paper here
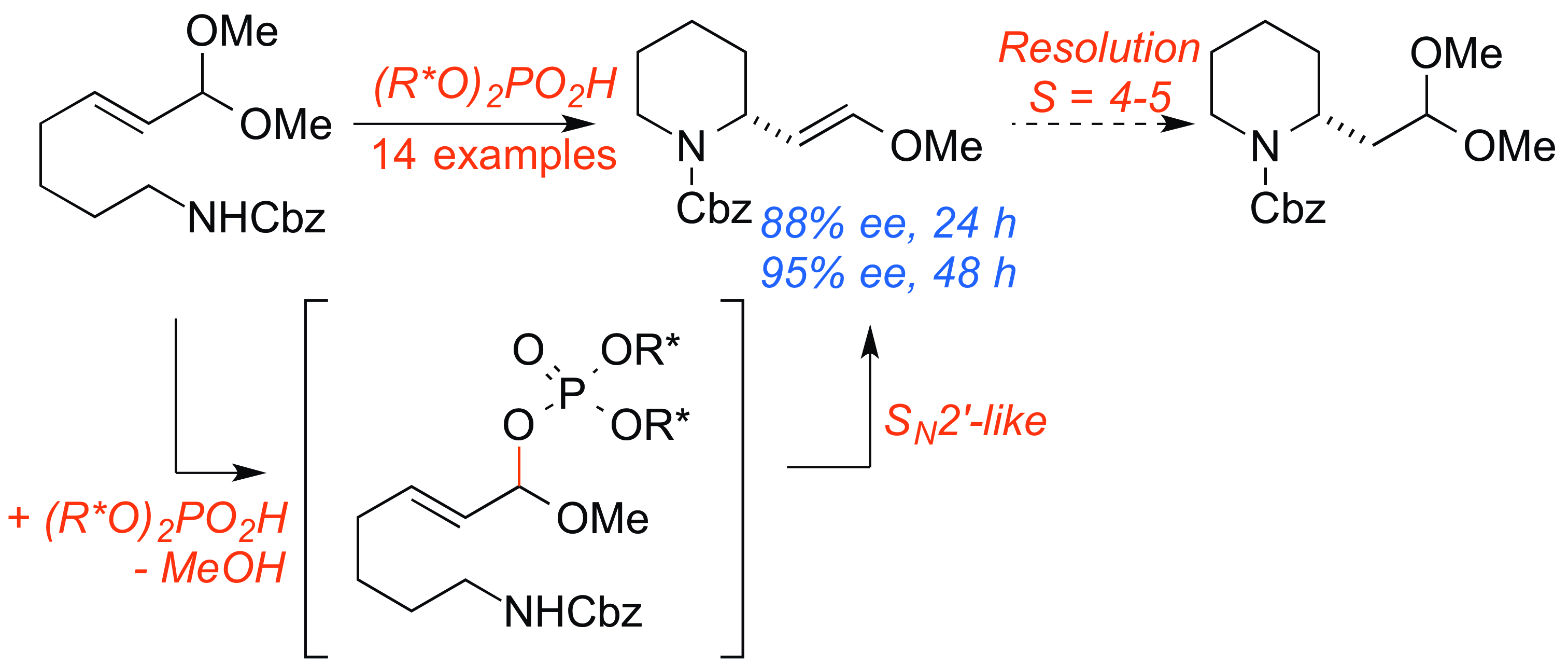
23. Sun, Z.; Winschel, G. A.; Zimmerman, P.;* Nagorny, P.* "Enantioselective Synthesis of Piperidines through the Formation of Chiral Mixed Phosphoric Acid Acetals: Experimental and Theoretical Studies" Angew. Chem. Int. Ed. 2014, 53, 11194. (selected as a Hot Paper by the editorial office of Angewandte Chemie, Highlighted in Synfacts). Access paper here
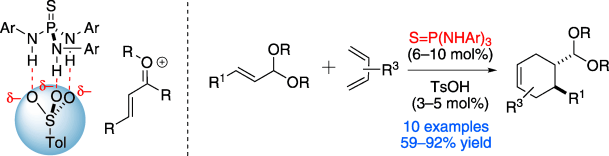
22. Borovika, A;* Tang, P.-I.*; Klapman, S.; Nagorny, P. "Thiophosphoramide-Based Cooperative Catalysis for the Bronsted Acid-Promoted Ionic Diels-Alder Reactions" Angew. Chem. Int. Ed.2013, 52, 13424. (selected as a Hot Paper by the editorial office of Angewandte Chemie; highlighted in Synfacts and ChemInform) Access paper here
21. Mensah, E.; Camasso, N.; Kaplan, W.; Nagorny, P. "Chiral Phosphoric Acid-Directed Regioselective Acetalization of Carbohydrate-Derived 1,2-diols" Angew. Chem. Int. Ed. 2013, 52, 13939. Access paper here
20. Larsen, B. J.; Sun, Z.; Nagorny, P. "Synthesis of Eukaryotic Translation Elongation Inhibitor Lactimidomycin via Zn(II)-mediated Horner-Wadsworth-Emmons Macrocyclization" Org.Lett.2013, 15, 2998. Access paper here
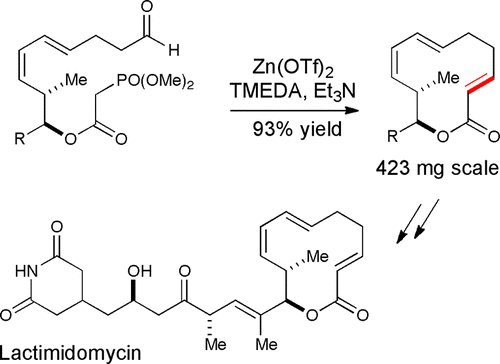
19. Borovika, A.; Nagorny, P. "Chiral Br�nsted Acid-catalyzed Enantioselective Ionic [2+4] Cycloadditions" Tetrahedron (Special issue in honor of Professor Melanie S. Sanford) 2013, 69, 5719. Access paper here
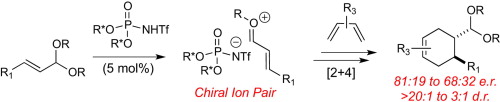
18. Nagorny, P.; Sun, Z.; Winschel, G. A. "Chiral Phosphoric Acid-Catalyzed Stereoselective Spiroketalizations" Synlett (Synpacts) 2013, 24, 661. Access paper here
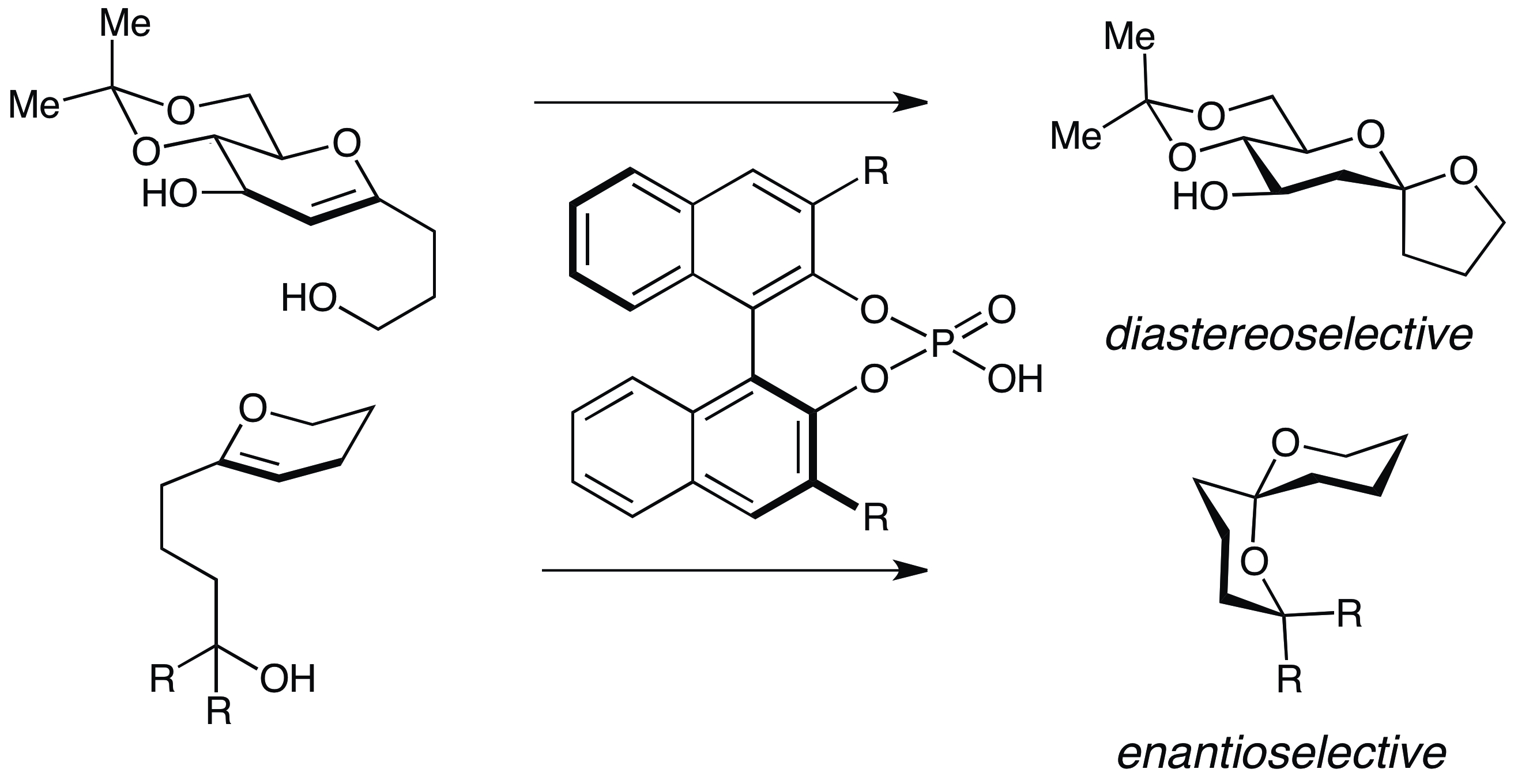
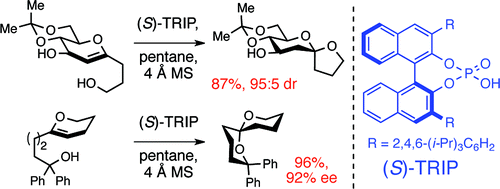
17. Sun, Z.; Winschel, G. A.; Borovika, A.; Nagorny, P. "Chiral Phosphoric Acid-Catalyzed Enantioselective and Diastereoselective Spiroketalizations" J. Am. Chem. Soc. 2012, 134, 8074.(Highlighted in Synfacts and ChemInform) Access paper here
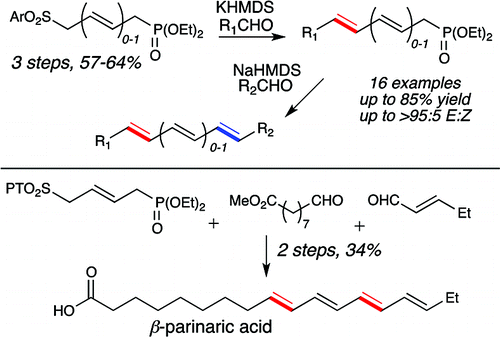
16. Cichowicz, N.; Nagorny, P. "Synthesis of Conjugated Polyenes via Sequential Condensation of Sulfonylphosphonates and Aldehydes" Org. Lett. 2012, 14, 1058.Access paper here
15. Borovika, A.; Nagorny, P. "Recent Advances in the Synthesis of Natural 2-deoxy-b-glycosides" J. Carb. Chem. 2012, 31, 255. (listed as one of the most viewed articles of J. Carb. Chem.)Access paper here
Undergraduate, Graduate and Postdoctoral Work:
14. Aussedat, B.; Fasching, B.; Johnston, E.; Sane, N.; Nagorny, P.; Danishefsky, S. J. "Total synthesis of the ?-subunit of the human glycoprotein hormones (hGPH): toward fully synthetic homogeneous human follicle-stimulating hormone (hFSH)" J. Am. Chem. Soc. 2012, 134, 3532-3541.
13. Nagorny, P.*; Sane, N.*; Fasching, B.; Aussedat, B.; Danishefsky, S. J. "Probing the frontiers of glycoprotein synthesis: the fully elaborated ?-subunit of the human follicle stimulating hormone" Angew. Chem. Int. Ed. 2011, 51, 975-979. *Equally contributing authors.
12. Lecomte, N.; Njardarson, J.; Nagorny, P.; Yang, G.; Downey, R.; Ouerfelli, O.; Moore, M.; Danishefsky, S. J. "Emergence of Potent Inhibitors of Metastasis in Lung Cancer via Syntheses Based on Migrastatin" Proc. Natl. Acad. Sci. USA 2011, 108, 15074-15078.
11. Nagorny, P.; Krauss, I.; Njardarson, J. T.; Perez, L.; Gaul, C.; Yang, G.; Ouerfelli, O.; Danishefsky, S. J. "Confirmation of the structure of synthetic derivatives of migrastatin in the light of recently disclosed crystallographically based claims" Tetrahedron Lett. 2010, 51(30), 3873-3875.
10. Oskarsson, T.; Nagorny, P.; Krauss, I. J.; Perez, L.; Mandal, M.; Yang, G.; Ouerfelli, O.; Xiao, D.; Moore, M. A. S.; Massague, J.; Danishefsky, S. J. "Promising Cell-Migration Inhibitors for Treatment of Tumor Metastasis: In Vivo and Mechanistic Studies on the Migrastatin Core Ether Analogs" J. Am. Chem. Soc. 2010, 132(9), 3224-3228.
9. Rao, Y.; Li, X.; Nagorny, P.; Hayashi J.; Danishefsky, S. J. "A Simple Method for the Conversion of Carboxylic Acids into Thioacids with Lawesson's Reagent" Tetrahedron Lett. 2009, 50(48), 6684-6686.
8. Nagorny, P.; Kim, W. H.; Wan, Q.; Lee, D.; Danishefsky, S. J. "On the Emerging Role of Chemistry in the Fashioning of Biologics: Synthesis of a Bidomainal Fucosyl GM1-Based Vaccine for the Treatment of Small Cell Lung Cancer" J. Org. Chem. 2009, 74(15), 5157-5162.
7. Nagorny, P.; Fasching, B.; Li, X.; Chen, G.; Aussedat, B.; Danishefsky, S. J. � Toward Fully Synthetic Homogeneous ?-Human Follicle-Stimulating Hormone (b-hFSH) with a Biantennary N-Linked Dodecasaccharide. Synthesis of b-hFSH with Chitobiose Units at the Natural Linkage Sites" J. Am. Chem. Soc. 2009, 131(16), 5792-5799.
6. Evans, D. A.; Nagorny, P.; McRae, K. J.; Reynolds, D. J.; Sonntag, L.-S.; Vounatsos, F.; Xu, R. "Enantioselective Synthesis of Oasomycin A, Part I. Synthesis of the C1-C12 and C13-C28 Subunits,"Angew. Chem. Int. Ed. 2007, 46, 537-540.
5. Evans, D. A.; Nagorny, P.; Reynolds, D. J.; McRae, K. J. "Enantioselective Synthesis of Oasomycin A, Part II. Synthesis of the C29-C46 Subunit," Angew. Chem. Int. Ed. 2007, 46, 541-544.
4. Evans, D. A.; Nagorny, P.; McRae, K. J.; Reynolds, D. J.; Sonntag, L.-S.; Vounatsos, F.; Xu, R. "Enantioselective Synthesis of Oasomycin A, Part III. Fragment Assembly and Conformation of Structure," Angew. Chem. Int. Ed. 2007, 46, 545-548.
3. Evans, D. A.; Nagorny, P.; Xu, R. "Ceric Ammonium Nitrate Promoted Oxidation of Oxazoles,"Org. Lett. 2006, 8, 5669-5671.
2. Blakemore, P.R.; Browder, C.C.; Hong, J.; Lincoln, C.M.; Nagornyy, P.A.; Robage, L.A.; Wardrop, D.J.; White, J.D. "Total Synthesis of Polycavernoside A, A Lethal Toxin of the Red Alga Polycavernosa tsudai," J. Org. Chem. 2005, 70, 5449-5460.
1. White, J.D.; Blakemore, P.R.; Browder, C.C.; Hong, J.; Lincoln, C.M.; Nagornyy, P.A.; Robage, L.A.; Wardrop, D.J. "Total Synthesis of the Marine Toxin Polycavernoside A via Selective Macrolactonization of a Trihydroxy Carboxylic Acid," J. Am. Chem. Soc. 2001, 123, 8593-8595.
Patents and Patent Applications:
Nagorny, P.; Arguelles, A. J.; Sun, S. "Spiroketal-Based C2-Symmetric Scaffold for Asymmetric Catalysis". U.S. Patent No. 10,565,015 (February 18, 2020).
Research Areas(s)
- Organic Chemistry
- Organocatalysis
- Asymmetric catalysis
- Total synthesis of natural products
- Carbohydrate Chemistry
- Medicinal Chemistry
Award(s)
- 2015 Amgen Young Investigator Award
- 2014 William Roush Award (University of Michigan)
- 2014 Alfred P. Sloan Research Fellowship
- 2014 NSF Career Award
- 2012 Thieme Chemistry Journal Award
- NIH Ruth L. Kirschstein Postdoctoral Fellowship (MSKCC)
- Eli Lilly Organic Chemistry Fellowship (Harvard University)
- Christensen Award (Harvard University)
- ACS Award in Analytical Chemistry (Oregon State University)
- Merck Index Award (Oregon State University)
About
Research in my group is focused on the areas of organic synthesis and catalysis with the long-term goals of (1) developing and exploring new catalytic transformations with an emphasis on organocatalytic transformations, and (2) developing concise syntheses of bioactive natural products (NPs) and using these syntheses as a platform for designing new therapeutic agents for the treatment of human diseases. These goals represent highly synergistic areas. Access to new catalytic transformations could significantly enhance the preparation and evaluation of bioactive NPs, which is of great importance to the field of drug discovery as more than 50% of approved therapeutic agents are derived from or mimic NPs. At the same time, the synthesis of bioactive NPs helps to identify the shortcomings of the existing synthetic methods and drives the development of new catalytic reactions. The students in my group are trained in various areas of organic synthesis and catalysis as well as in the areas of medicinal and computational chemistry.
Focus Area 1. Developing Concise Asymmetric Syntheses of Natural Products. Bioactive natural products have served as an essential and continuous source of therapeutic agents for the treatment of various human diseases. The ability to synthetically generate bioactive natural products and their derivatives has been of great importance to the field of drug discovery. Despite some great advances in the synthesis of certain classes of natural products, many important types of medicinally valuable natural products and their derivatives are still not readily available by synthesis. We have identified cardiotonic steroids, bioactive diterpenes, and glutarimide-based macrolactones as important families of bioactive natural products, biological studies which would greatly benefit from having simple and concise syntheses of these molecules. A primary goal for our group in these studies is to develop general, scalable, and modular approaches to the aforementioned classes of natural products and edit the structure of these compounds to improve their biological properties. Some of our recent progress in this area is summarized in the publications below (publications 2, 6, 9, 10, and 16).
Focus Area 2. Selective Chiral Catalyst-Controlled Functionalization of Natural Products. One of our major research programs is focused on discovering new catalytic methods directed to simplify the chemical functionalization of complex natural products using small molecule-based chiral catalysts rather than enzymes. In our recently published efforts, we have explored the use of chiral Brønsted acids as simple organic catalysts mimicking the function of glycosyltransferases. Our group has developed chiral phosphoric acid-based catalysts that can promote highly site-selective acetalization of monosaccharide-derived polyols. Building on these results, we have achieved chiral Brønsted acid-controlled site-selective glycosylations of 14-membered macrolide-derived triols and tetraols and regioselective desymmetrizative glycosylation of 6-deoxystreptamine derivatives. In our collaborative efforts, we have also demonstrated that these reactions proceed through the covalently linked glycosyl phosphates, which also provides an opportunity to design various stoichiometric solid-supported reagents and catalysts for site-selective glycosylation. These methodologies may provide direct access to glycosylated derivatives of complex natural polyols thus significantly simplifying the preparation of such derivatives. In addition to glycosylation, other types of functionalizations are currently being explored using organic Bronsted acids as catalysts.
Focus Area 3. Exploring Organocatalytic Transformations of Oxocarbenium Ions. The development of stereoselective and regioselective transformations of organic molecules represents one of the fundamental challenges in modern organic synthesis, and a lack of selective methods may significantly affect the availability and purity of biologically active NPs and therapeutic agents. Among such gaps is the lack of catalyst-controlled transformations involving oxocarbenium ions. The chemistry of oxocarbenium ions is central to the preparation of many medicinally important classes of compounds such as glycosides, acetals, spiroketals as well as oxygen-containing heterocyclic compounds. The therapeutic properties of these compounds strongly depend on the stereochemical features and position of the acetal linkage within the molecule. However, synthetic installation of these features is particularly challenging for the reactions involving oxocarbenium ions. This part of our research program focuses on identifying new techniques to control the selective formation of acetals by harnessing the reactivity of oxocarbenium ions using chiral catalysts. Some of our recent progress in this area is summarized in the publications below.
Focus Area 4. Asymmetric catalysis, chiral ligand and catalyst design, and new synthetic method development. The design and development of new chiral ligands and catalysts have been one of the important directions in our group. These studies are highly synergistic with the other research directions described above as asymmetric catalysis has played the central role in the majority of the synthetic and methodological studies in which our group has been involved. Based on our experiences in spiroketalization studies, our group has designed a new class of spiroketal-based chiral scaffolds termed SPIROLs. The SPIROL-based ligands have demonstrated excellent performance in a variety of Pd-, Rh- and Ir-catalyzed transformations while being significantly more accessible than other types of spirocyclic ligands. Our group continues further development and investigation of the SPIROL-based ligands, in particular, in the context of the cardiotonic steroid and catalyst-controlled natural product functionalization studies. This includes the exploration of SPIROL-based ligands for the enantioselective Robinson-annulation leading to quaternary stereocenter-containing polycyclic molecules recently reported in our group as well as site-selective diastereoselective functionalization of natural products.
In addition, our group has contributed to improving the methods available for the preparation and characterization of the existing chiral scaffolds and catalysts as well as designing new recyclable chiral Brønsted acid-based organocatalysts that are immobilized on the polymer matrix. We are also interested in designing new methods and strategies that would improve access to important building blocks and functionalities.
Submitted for review and preprints:
A) Krishnarjuna, B.; Sharma, G.; Hiiuk, V.; Struppe, J.; Nagorny, P.; Ivanova, M.; Ramamoorthy, A. "Nanodisc reconstitution and characterization of amyloid-b-precursor protein C99" 2023, Submitted. Access PrePrint Here.
Published Work:
60) Tichvon, C.; Zviagin, E.; Surma, Z.; Nagorny, P.* "Concise Synthesis of Bufadienolide Cinobufagin via Late-Stage Singlet Oxygen Oxidation/Rearrangement Approach" Org. Lett. 2024, 26, 2445.
59) Zhelavskyi, O.; Parikh, S.; Staples, R. J.; Zimmerman, P. M.,* Nagorny, P.* "Green Light Promoted Iridium(III)/Copper(I)-Catalyzed Addition of Alkynes to Aziridinoquinoxalines Through the Intermediacy of Azomethine Ylides" Angew. Chem. Int. Ed. 2024, e202318876, DOI: 10.1002/anie.202318876
Access the ChemRxiv PrePrint here.

58) Rutkoski, R.; Debarba, L. K.; Stilgenbauer, L.; Rosenthal, T.; Sadagurski, M.;* Nagorny, P.* "Selective (a)-L-Rhamnosylation and Neuroprotective Activity Exploration of Cardiotonic Steroids" ACS Med. Chem. Lett. 2024, 15, 280.

57. Rutkoski, R.; Arguelles, A. J.; Huang, Q.; Nagorny, P.* "Development of Recyclable Polystyrene Supported Phosphonic Acid Resins for Carbohydrate Immobilization and Glycosylation" J. Org. Chem. 2023, 88, 16467. Access Article Here.

56. Jhang, Y.-J.#; Zhelavskyi, O.#; Nagorny, P.*; “Enantioselective Parallel Kinetic Resolution of Aziridine-Containing Quinoxalines via Chiral Phosphoric Acid-Catalyzed Transfer Hydrogenation”Org.Lett. 2023, 25, 7721. Access Article Here

55. Zhelavskyi, O.; Jhang, Y.-J.; Nagorny, P.*; “Asymmetric Transfer Hydrogenation of Heterocyclic Compounds in Continuous Flow Using an Immobilized Chiral Phosphoric Acid as the Catalyst” Synthesis 2023, 55, 2361 (Special Edition Dedicated to D. A. Evans). Access Article Here

54. Carney, N.; Perry, N.; Garabedian, J.; Nagorny, P. "Development of a-Selective Glycosylation with L-Oleandral and Its Application to the Total Synthesis of Oleandrin" Org. Lett. 2023, 6, 966. Access Article Here

53. Eid, S.; Zerbes, T.; Williams, D.; Wang, X. Z.; Sackmann, C.; Meier, S.; Dulin, N. O.; Nagorny, P. Schmitt-Ulms, G. “Indentification of a Cardiac Glycoside Exhibiting Favorable Brain Bioavailability and Potency for Reducing Levels of the Cellular Prion Protein” Int. J. Mol. Sci. 2022, 23, 14823. Access Article Here.
52. Kim, S.; Nagorny, P. "Electrochemical Fluorination with Sulfur(VI) Hexafluoride for the Synthesis of Glycosyl Fluoride" Org. Lett. 2022, 24, 2294. Access Article Here.

51. Diaz, N.; Nagorny, P. "(11bR)-4-Hydroxy-2,6-bis[2,4,6-tris(1-methylethyl)phenyl]-dinaphtho[2,1-d: 1',2'-f][1,3,2]dioxaphosphepin-4-oxide, [(R)-TRIP] & (11bS)-4-Hydroxy-2,6-bis[2,4,6-tris(1-methylethyl)phenyl]-dinaphtho[2,1-d: 1',2'-f][1,3,2]dioxaphosphepin-4-oxide, [(S)-TRIP]" (Update) Encyclopedia of Reagents in Organic Synthesis 2022. Access Article Here.
50. Sun, S.; Fejedelem, Z.; Song, S.; Nagorny, P. "Synthesis of (((1R,3S,3'S)-3,3'-diethyl-3H,3'H-1,1'-spirobi[isobenzofuran]-7,7'-diyl)bis(oxy))bis(diphenylphosphane) Org. Syn. 2022, 99, 190.
49. Sun, S.; Nagorny, P. "Synthesis of Chiral Aziridine Ligands for Asymmetric Alkylation with Alkylzincs: diphenyl((S)-1-((S)-1-phenylethyl)aziridin-2-yl)methanol" Org. Syn. 2021, 98, 446. Access Article Here.
48. Wang, S.; Zhelavskyi, O.; Lee, J.; Arguelles, A. J.; Khomutnyk, Y.; Mensah, E. A.; Guo, H.; Hourani, R.; Zimmerman, P. M.; Nagorny, P. "Studies of catalyst-controlled regioselective acetalization and its application to single-pot synthesis of differentially protected saccharides" J. Am. Chem. Soc. 2021, 143, 18592. Access Article Here
Pre Print: ChemRxiv. PrePrint. Access Article Here.

47. Sun, S.; Nagorny, P. " Synthesis and Evaluation of C2-Symmetric SPIROL-based bis-Oxazoline Ligands" Symmetry 2021, 13, 1667. (Special Edition dedicated to Symmetric and Asymmetric Total Synthesis). Access Article Here.

46. Fejedelem, Z.; Carney, N.; Nagorny, P. "Synthesis of cardiotonic steroids oleandrigenin and rhodexin B" J. Org. Chem. 2021, 86, 10249. Access Article Here.

45. Kim, S.; Khomutnyk, Y.; Bannykh, A.; Nagorny, P. "Synthesis of glycosyl fluorides by photochemical fluorination with sulfur(VI) hexafluoride" Org. Lett. 2021, 23, 190. (ACS Editor's Choice). Access this manuscript here.

44. Sun, S.; Nagorny, P. "Exploration of chiral diastereomeric spiroketal (SPIROL)-based phosphinite ligands in asymmetric hydrogenation of heterocycles" Chem. Commun. 2020, 56, 8432.

43. Wang, S.; Arguelles, A. J.; Tay, J.-H.; Hotta, M.; Zimmerman, P. M.*; Nagorny, P.* "Experimental and computational studies on regiodivergent chiral phosphoric acid catalyzed cycloisomerization of mupirocin methyl ester" Chem. Eur. J. 2020, 26, 4583 (Hot Article). Access Paper Here.

42. Khatri, H. R.; Carney, N.; Rutkoski, R.; Bhattarai, B.; Nagorny, P. "Recent progress in steroid synthesis triggered by the emergence of new catalytic methods" Eur. J. Org. Chem. 2020, 21, 755 (Invited Minireview); Access Paper Here.

41.Khatri, H. R.; Bhattarai, B.; Kaplan, W.; Li, Z.; Long, M. J. C.;* Aye, Y.;* Nagorny, P.* "Modular total synthesis and cell-based anticancer activity evaluation of ouabagenin and other cardiotonic steroids with varying degrees of oxygenation" J. Am. Chem. Soc. 2019, 141, 4849. Access Paper Here.

40.Tay, J.-H.; Dorokhov, V.; Wang, S.; Nagorny, P. "Regioselective Single Pot C3-Glycosylation of Strophanthidol Using Methylboronic Acid as a Traceless Protecting Group" J. Antibiotics, 2019, 72, 437 (Invited Contribution to the Special Issue Dedicated to Professor Samuel Danishefsky). Access paper here.

39.Kearney, S. E. et al. "Canvass: A Crowd-Sourced, Natural-Product Screening Library for Exploring Biological Space" ACS Cent. Sci., 2018, 4, 1727. Access paper here.

38. Lee, J.; Wang, S.; Callahan, M.; Nagorny, P.* "Copper(II)-catalyzed tandem decarboxylative Michael/Aldol reactions leading to the formation of functionalized cyclohexenones" Org. Lett., 2018, 20, 2067. Access paper here.

37. Arguelles, A. J.; Sun, S.; Budaitis, B.; Nagorny, P.* "Design, synthesis, and application of chiral C2-symmetric spiroketal-containing ligands in transition-metal catalysis" Angew. Chem. Int. Ed., 2018, 57, 5325. Access paper here.

36. Bhattarai, B.; Nagorny, P.* "Enantioselective total synthesis of cannogenol-3-O-a-L-rhamnoside via sequential Cu(II)-catalyzed Michael addition/intramolecular aldol cyclization reactions" Org.Lett., 2018, 20, 154. Access paper here.

35. Lee, J.; Borovika, A.; Khomutnyk, Y.; Nagorny, P.* "Chiral phosphoric acid-catalyzed desymmetrizative glycosylation of 2-deoxystreptamine and its application to aminoglycoside synthesis" Chem. Commun., 2017, 53, 8976. (themed issue "Site Selective Molecular Transformations"). Access paper here.

34. Tay, J.-H.; Nagorny, P.* "Bronsted Acid Catalysts Derived from Phosphorous" Non-nitregenous Organocatalysis, Chapter 3, 2017 (edited by Andrew Harned), In Production.
33. Tay, J.-H.; Arguelles, A. J.; DeMars II, M. D.; Zimmerman, P. M.;* Sherman, D. H.;* Nagorny, P.* "Regiodivergent Glycosylations of 6-deoxy-Erythronolide B and Oleandomycin-derivied Macrolides Enabled by Chiral Acid Catalysis" J. Am. Chem. Soc. 2017, 139, 8570. Access paper here.

32. Nagorny, P.;* Sun, Z.* "New Approaches to Organocatalysis Based on C–H and C–X bonding for Electrophilic Substrate Activation" Beilst. J. Org. Chem. 2016, 12, 2834. Access paper here.
31. Kaplan, W.; Khatri, H. R.; Nagorny, P. "Concise Enantioselective Total Synthesis of Cardiotonic Steroids 19-Hydroxysarmentogenin and Trewianin Aglycone" J. Am. Chem. Soc. 2016, 138, 7194. Access paper here.

30. Nagorny, P.; Cichowicz, N. "New Strategy Based on Sequential Michael/Aldol Reactions for the Asymmetric Synthesis of Cardenolides" Strategies and Tactics in Organic Synthesis 12 (Book chapter), Volume 12, Chapter 9, 2016 (edited by Michael Harmata), 238-266.
29. Khomutnyk, Y. Y.; Arguelles, A. J.; Winschel, G. A.; Sun, Z.; Zimmerman, P. M.;* Nagorny, P.* "Studies of the Mechanism and Origins of Enantioselectivity for the Chiral Phosphoric Acid-Catalyzed Stereoselective Spiroketalization" J. Am. Chem. Soc. 2016, 138, 444.Access paper here

28. Tay, J.-H.; Nagorny, P.* "31P NMR-based Method for Determining Enantiopurity of Chiral Phosphoric Acids, and Its Application to the BINOL- and H8-BINOL-based Chiral Phosphoric Acid Thermal Racemization Studies" Synlett (Invited submission for Asymmetric Phosphoric Acid Catalysis Cluster) 2016, 27(04), 551-554. Access paper here.

27.Cichowicz, N.; Kaplan, W.; Khomutnik, I.; Bhattarai, B.; Sun, Z.; Nagorny, P.* "Concise enantioselective synthesis of oxygenated steroids via sequential copper(II)-catalyzed Michael addition/intramolecular aldol cyclization reactions" J. Am. Chem. Soc. 2015, 137, 14341. (One of the top 20 most downloaded JACS publications in the month of December). Access paper here.

26. Larsen, B. J.; Sun, Z.; Lachacz, E.; Khomutnik, I.; Soellner, M. B.*; Nagorny, P.* "Synthesis and Biological Evaluation of Lactimidomycin and its Analogs" Chem. Eur. J. 2015, 21, 19159.Access paper here.

25. Tay, J.-H.; Arguelles, J. A.; Nagorny, P.* "Direct Interconversion of BINOL and H8-BINOL-based Chiral Bronsted Acids Using Single Step Red/Ox Manipulations" Org. Lett.2015, 17, 3774. Access paper here

24. Bhattarai, B.; Tay, J.-H.; Nagorny, P.* "Thiophosphoramides as Cooperative Catalysts for Copper-Catalyzed Arylation of Carboxylates with Diaryliodonium Salts" Chem. Commun. 2014, 51, 5398. Access paper here
23. Sun, Z.; Winschel, G. A.; Zimmerman, P.;* Nagorny, P.* "Enantioselective Synthesis of Piperidines through the Formation of Chiral Mixed Phosphoric Acid Acetals: Experimental and Theoretical Studies" Angew. Chem. Int. Ed. 2014, 53, 11194. (selected as a Hot Paper by the editorial office of Angewandte Chemie, Highlighted in Synfacts). Access paper here

22. Borovika, A;* Tang, P.-I.*; Klapman, S.; Nagorny, P. "Thiophosphoramide-Based Cooperative Catalysis for the Bronsted Acid-Promoted Ionic Diels-Alder Reactions" Angew. Chem. Int. Ed.2013, 52, 13424. (selected as a Hot Paper by the editorial office of Angewandte Chemie; highlighted in Synfacts and ChemInform) Access paper here

21. Mensah, E.; Camasso, N.; Kaplan, W.; Nagorny, P. "Chiral Phosphoric Acid-Directed Regioselective Acetalization of Carbohydrate-Derived 1,2-diols" Angew. Chem. Int. Ed. 2013, 52, 13939. Access paper here

20. Larsen, B. J.; Sun, Z.; Nagorny, P. "Synthesis of Eukaryotic Translation Elongation Inhibitor Lactimidomycin via Zn(II)-mediated Horner-Wadsworth-Emmons Macrocyclization" Org.Lett.2013, 15, 2998. Access paper here

19. Borovika, A.; Nagorny, P. "Chiral Br�nsted Acid-catalyzed Enantioselective Ionic [2+4] Cycloadditions" Tetrahedron (Special issue in honor of Professor Melanie S. Sanford) 2013, 69, 5719. Access paper here

18. Nagorny, P.; Sun, Z.; Winschel, G. A. "Chiral Phosphoric Acid-Catalyzed Stereoselective Spiroketalizations" Synlett (Synpacts) 2013, 24, 661. Access paper here

17. Sun, Z.; Winschel, G. A.; Borovika, A.; Nagorny, P. "Chiral Phosphoric Acid-Catalyzed Enantioselective and Diastereoselective Spiroketalizations" J. Am. Chem. Soc. 2012, 134, 8074.(Highlighted in Synfacts and ChemInform) Access paper here

16. Cichowicz, N.; Nagorny, P. "Synthesis of Conjugated Polyenes via Sequential Condensation of Sulfonylphosphonates and Aldehydes" Org. Lett. 2012, 14, 1058.Access paper here

15. Borovika, A.; Nagorny, P. "Recent Advances in the Synthesis of Natural 2-deoxy-b-glycosides" J. Carb. Chem. 2012, 31, 255. (listed as one of the most viewed articles of J. Carb. Chem.)Access paper here
Undergraduate, Graduate and Postdoctoral Work:
14. Aussedat, B.; Fasching, B.; Johnston, E.; Sane, N.; Nagorny, P.; Danishefsky, S. J. "Total synthesis of the ?-subunit of the human glycoprotein hormones (hGPH): toward fully synthetic homogeneous human follicle-stimulating hormone (hFSH)" J. Am. Chem. Soc. 2012, 134, 3532-3541.
13. Nagorny, P.*; Sane, N.*; Fasching, B.; Aussedat, B.; Danishefsky, S. J. "Probing the frontiers of glycoprotein synthesis: the fully elaborated ?-subunit of the human follicle stimulating hormone" Angew. Chem. Int. Ed. 2011, 51, 975-979. *Equally contributing authors.
12. Lecomte, N.; Njardarson, J.; Nagorny, P.; Yang, G.; Downey, R.; Ouerfelli, O.; Moore, M.; Danishefsky, S. J. "Emergence of Potent Inhibitors of Metastasis in Lung Cancer via Syntheses Based on Migrastatin" Proc. Natl. Acad. Sci. USA 2011, 108, 15074-15078.
11. Nagorny, P.; Krauss, I.; Njardarson, J. T.; Perez, L.; Gaul, C.; Yang, G.; Ouerfelli, O.; Danishefsky, S. J. "Confirmation of the structure of synthetic derivatives of migrastatin in the light of recently disclosed crystallographically based claims" Tetrahedron Lett. 2010, 51(30), 3873-3875.
10. Oskarsson, T.; Nagorny, P.; Krauss, I. J.; Perez, L.; Mandal, M.; Yang, G.; Ouerfelli, O.; Xiao, D.; Moore, M. A. S.; Massague, J.; Danishefsky, S. J. "Promising Cell-Migration Inhibitors for Treatment of Tumor Metastasis: In Vivo and Mechanistic Studies on the Migrastatin Core Ether Analogs" J. Am. Chem. Soc. 2010, 132(9), 3224-3228.
9. Rao, Y.; Li, X.; Nagorny, P.; Hayashi J.; Danishefsky, S. J. "A Simple Method for the Conversion of Carboxylic Acids into Thioacids with Lawesson's Reagent" Tetrahedron Lett. 2009, 50(48), 6684-6686.
8. Nagorny, P.; Kim, W. H.; Wan, Q.; Lee, D.; Danishefsky, S. J. "On the Emerging Role of Chemistry in the Fashioning of Biologics: Synthesis of a Bidomainal Fucosyl GM1-Based Vaccine for the Treatment of Small Cell Lung Cancer" J. Org. Chem. 2009, 74(15), 5157-5162.
7. Nagorny, P.; Fasching, B.; Li, X.; Chen, G.; Aussedat, B.; Danishefsky, S. J. � Toward Fully Synthetic Homogeneous ?-Human Follicle-Stimulating Hormone (b-hFSH) with a Biantennary N-Linked Dodecasaccharide. Synthesis of b-hFSH with Chitobiose Units at the Natural Linkage Sites" J. Am. Chem. Soc. 2009, 131(16), 5792-5799.
6. Evans, D. A.; Nagorny, P.; McRae, K. J.; Reynolds, D. J.; Sonntag, L.-S.; Vounatsos, F.; Xu, R. "Enantioselective Synthesis of Oasomycin A, Part I. Synthesis of the C1-C12 and C13-C28 Subunits,"Angew. Chem. Int. Ed. 2007, 46, 537-540.
5. Evans, D. A.; Nagorny, P.; Reynolds, D. J.; McRae, K. J. "Enantioselective Synthesis of Oasomycin A, Part II. Synthesis of the C29-C46 Subunit," Angew. Chem. Int. Ed. 2007, 46, 541-544.
4. Evans, D. A.; Nagorny, P.; McRae, K. J.; Reynolds, D. J.; Sonntag, L.-S.; Vounatsos, F.; Xu, R. "Enantioselective Synthesis of Oasomycin A, Part III. Fragment Assembly and Conformation of Structure," Angew. Chem. Int. Ed. 2007, 46, 545-548.
3. Evans, D. A.; Nagorny, P.; Xu, R. "Ceric Ammonium Nitrate Promoted Oxidation of Oxazoles,"Org. Lett. 2006, 8, 5669-5671.
2. Blakemore, P.R.; Browder, C.C.; Hong, J.; Lincoln, C.M.; Nagornyy, P.A.; Robage, L.A.; Wardrop, D.J.; White, J.D. "Total Synthesis of Polycavernoside A, A Lethal Toxin of the Red Alga Polycavernosa tsudai," J. Org. Chem. 2005, 70, 5449-5460.
1. White, J.D.; Blakemore, P.R.; Browder, C.C.; Hong, J.; Lincoln, C.M.; Nagornyy, P.A.; Robage, L.A.; Wardrop, D.J. "Total Synthesis of the Marine Toxin Polycavernoside A via Selective Macrolactonization of a Trihydroxy Carboxylic Acid," J. Am. Chem. Soc. 2001, 123, 8593-8595.
Patents and Patent Applications:
Nagorny, P.; Arguelles, A. J.; Sun, S. "Spiroketal-Based C2-Symmetric Scaffold for Asymmetric Catalysis". U.S. Patent No. 10,565,015 (February 18, 2020).
Research Areas(s)
- Organic Chemistry
- Organocatalysis
- Asymmetric catalysis
- Total synthesis of natural products
- Carbohydrate Chemistry
- Medicinal Chemistry
Award(s)
- 2015 Amgen Young Investigator Award
- 2014 William Roush Award (University of Michigan)
- 2014 Alfred P. Sloan Research Fellowship
- 2014 NSF Career Award
- 2012 Thieme Chemistry Journal Award
- NIH Ruth L. Kirschstein Postdoctoral Fellowship (MSKCC)
- Eli Lilly Organic Chemistry Fellowship (Harvard University)
- Christensen Award (Harvard University)
- ACS Award in Analytical Chemistry (Oregon State University)
- Merck Index Award (Oregon State University)