Fitness effects of altering gene expression noise in Saccharomyces cerevisiae
- All News
-
- Grad Student News
- Outreach News
- Search News
- Science Fun Facts
- Social Media
- Archived News
- Newsletters
- Expert Insights: Ben Hess on Importing Biological Materials
- U-M Herbarium Publication Spotlight: Dr. Thaís Vasconcelos and Dr. Aly Baumgartner Collaborate on Paper in New Phytologist
- U-M Museum of Zoology Publication Spotlight: Dr. Benjamin Winger's Study on Songbirds
- UMMZ Spotlight: Charlie Engelman Named to TIME’s "100 Most Influential Creators of 2025"
- Herbarium Spotlight: How AI is Transforming Specimen Transcription
- UMMZ Spotlight: A’liya Spinner is helping preserve the future of bees
- Meet the Researchers Driving Discovery Through the Biodiversity Exploration Fund
- EEB and U-M Museum of Natural History Celebrate ID Day
- All Events
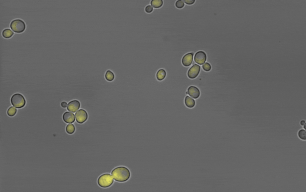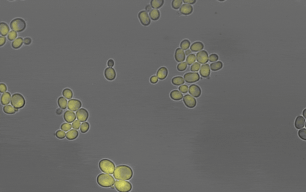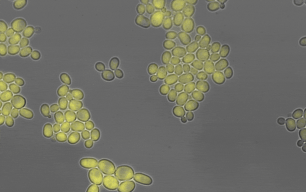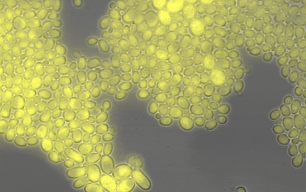
Members of the lab of Professor Patricia Wittkopp authored a paper in eLife titled “Fitness effects of altering gene expression noise in Saccharomyces cerevisiae,” with first author Fabien Duveau, a former postdoctoral fellow in her lab.
The paper was published with a corresponding eDigest that is used to enhance science communication about the paper. The eDigest follows, with the addition of a few quotes and information about the authors.
Single-celled organisms that reproduce by dividing, like yeast, can create whole populations of genetically identical cells. However, some differences will exist among such cells, even when they have all experienced the same environment. These differences are known as “noise.” By definition, noise is not caused by differences in DNA sequence, but some DNA sequences are noisier than others (i.e. they cause more differences among cells). Because the amount of noise can be under genetic control, noise could evolve due to natural selection.
Scientists often study noise at the level of gene expression – in other words, how many RNA or protein molecules are produced from each gene within each cell. Prior work has suggested that this type of noise can affect how often individual cells divide in a population, which is a component of that population’s fitness. Yet directly measuring these effects has proven challenging. Different studies have in the past reached opposite conclusions about whether a change in gene expression noise would increase or decrease fitness.
One major reason for the lack of clear results is that most mutations that alter gene expression noise also alter the average level of expression of that gene. To find DNA sequences that produced the same average amount of protein but different levels of expression noise, Duveau et al. compared the effects of hundreds of mutations in the DNA sequence regulating the expression of a gene in baker’s yeast. Experiments focused on 43 DNA sequences then showed that increased expression noise could either speed up or slow down the growth of the population by affecting how long it took each cell to divide. More specifically, the effects of increasing expression noise depended on the average amount of protein produced among the cells in the population. If the average expression level was close to the optimum amount at which cells divided as fast as possible, increasing expression noise reduced the growth of the whole population. If, however, the average protein level caused cells to divide slower than their maximum rate, increasing expression noise resulted in faster growth of the population as a whole.
Duveau et al. explain their results as follows: more expression noise in a population that is already making the optimal amount of protein can reduce fitness because it increases the fraction of that population making a suboptimal amount of the protein. However, when the average expression level is not optimal, more expression noise would mean more cells producing an amount of protein that is closer to the optimum and thus having higher fitness.
“Our prior work (Metzger et al. 2015) suggested that increasing expression noise for this gene in particular was bad (decreased fitness),” said Wittkopp. “It was therefore surprising to find that it could also be beneficial in some circumstances.
“The other thing that was exciting was that we were able to detect and measure the effects of changing expression noise on fitness. Prior work suggested that the fitness effects of changing noise might be too small to measure in the laboratory.”
These findings provide conceptual tools needed to understand how genetic variation affecting expression noise evolves. They could also help understand how expression noise might contribute to biological processes that depend upon cell division, such as diseases like cancer.
“One future direction is to see whether expression noise affecting other genes has similar consequences for fitness,” Wittkopp added. “There is also work to be done to more directly link dynamics in gene expression within individual cells to their division time.”
Duveau is a postdoc at the National Center for Scientific Research (CNRS Institute, Laboratory Matter and Complex Systems) in Paris, France. Coauthors are Andrea Hodgins-Davis, postdoctoral fellow, Wittkopp lab; Brian PH Metzger, former graduate student, currently postdoctoral fellow, The University of Chicago; Bing Yang, former graduate student, now postdoc, University of California, San Diego; Stephen Tryban and Tricia Lybrook, former undergraduate students; Elizabeth A Walker, former lab manager, now microbiology technician at Pfizer. Wittkopp is an Arthur F. Thurnau Professor and the Sally L. Allen Collegiate Professor and associate chair for graduate studies for the Department of Ecology and Evolutionary Biology.

