By Richard E. Etheridge (adapted for the WWW and updated by M. O'Brien and G. Schneider, May 1996)
Most of the larger museums and universities that maintain preserved collections of reptiles and amphibians have curators trained in the approved methods of preparing and maintaining an alcoholic collection. On the other hand, many individuals with a non-professional interest in natural history have the inclination and opportunity to obtain and preserve herpetological specimens but lack knowledge of the proper techniques. It is the purpose of this article to be of help to these persons, for even small collections casually assembled may be of great usefulness if the specimens are adequately labeled, well preserved, and fixed in a standard position.
Steps for the preservation of specimens for sclentific study are as follows:
- Euthanizing. Specimens should be euthanized in a way that will leave them undamaged and relaxed, and follows best practices set forth by the Institutional Animal Care and Use Committee (IACUC).
- Injection and slitting. Liquid preservatives must be introduced into the body cavity, limbs and tail, either by hypodermic injection or through slits.
- Fixing. While the specimens are still relaxed, they should be arranged in trays so that they will harden in the proper position.
- Labeling. Each specimen should be accompanied by certain data, either attached directly or entered in a notebook with a number corresponding to a numbered tag tied to the specimen.
- Storage. After specimens have been fixed in the proper position, they should be stored in liquid preservative for at least several days, after which they may be allowed to remain in the liquid, or transferred to plastic bags for temporary storage.
Preserving Solutions
Formalin: If at all possible, formalin should be used for injecting and fixing specimens. Formalin is the commercial name of a solution of formaldehyde gas (CH20) in water. It is available at drugstores and chemical supply houses in the United States at a strength af from 38% to 40%. In Latin American countries, formalin may be purchased in many drugstores under the name "Formol" or "Formolina". Formalin must be diluted with water before it is used as a preservative. A strength of 10% formalin is best for most purposes. If the original strength is 40%, it should be mixed at a ratio af nine parts water to one part formalin. The advantages of formalin over other preservatives are: it is inexpensive, it is generally available, a small bulk af concentrated stock solution may be diluted as needed, and specimens almost never decay in it. Its principal disadvantages are: it has a very irritating odor, it is very poisonous and may cause skin irritation or rash, it has a tendency to make specimens become brittle if the solution is too strong, and tends to fade out certain colors rapidly, and it must be stored in rustproof containers. (Buffering of the 10% solution is recommended as formalin is slightly acidic. One buffering system that may be used is a mixture of monobasic and dibasic Sodium Phosphate, at 13 gm/gallon [Monobasic] and 24 gm/gallon [Dibasic]).
Alcohol. There is a high federal tax on ethyl alcohol which makes it a very expensive preservative unless it can be purchased by or through a university, museum or another such institution. It is usually sold at a strength of 95% (190 proof). For injection and fixing it should be used at full strength. For storage of reptiles it should be used in the proportion of 3 parts 95% alcohol to 1 part water. Alcohol which has been stored in open containers loses its strength rapidly due to evaporation. Strength may be tested with an alcoholometer. Specimens which have been fixed in alcohol should be carefully watched for signs of rotting. Alcoholic beverages, shaving lotions and Bay Rum contain ethyl alcohol. They should be used only in an emergency and without dilution. Liquor which is 100 proof is only 50% ethyl alcohol.
Preparation: If specimens are to be made permanently immune to decomposition, it is necessary that liquid preservative be introduced into the body cavity, limbs and tail within as short a time as possible after the animals have been killed. This may be accomplished either by injection (with a hypodermic syringe) or by making deep cuts with a sharp scalpel, razor blade or scissors. The most satisfactory way is by injection. A ten or twenty cc. syringe with a needle lock and several needles (guages 18 to 26) will serve to inject most specimens.
Frogs and Toads: Injection should be made through the belly, directly into the body cavity. If the body is puffed with air, it should be deflated by gently squeezing with the fingers. Very small frogs require only a few drops of preservative; frogs two or three inches long only a few cc. Introduce only enough preservative required to make the specimen look natural--it should not look bloated. It is not necessary to inject the legs of any but the largest frogs. If equipment for injection is not available, a single slit may be made in the abdomen, to one side of the midline. The slit should be deep enough to allow free access of the preservative into the body cavity.
Salamanders. Most salamanders do not require injection or slitting. If your specimens look "caved in" a small amount of preservative may be injected into the body cavity, or a single slit made in the abdomen to permit preservative.
Tadpoles. Tadpoles and small salamander larvae should always be preserved in 10% formalin, never in alcohol. Simply drop the tadpoles into formalin while they are still alive. Be sure there is enough preservative to cover them and avoid overcrowding. After 24 hours all the liquid should be drained off and replaced with fresh formalin.
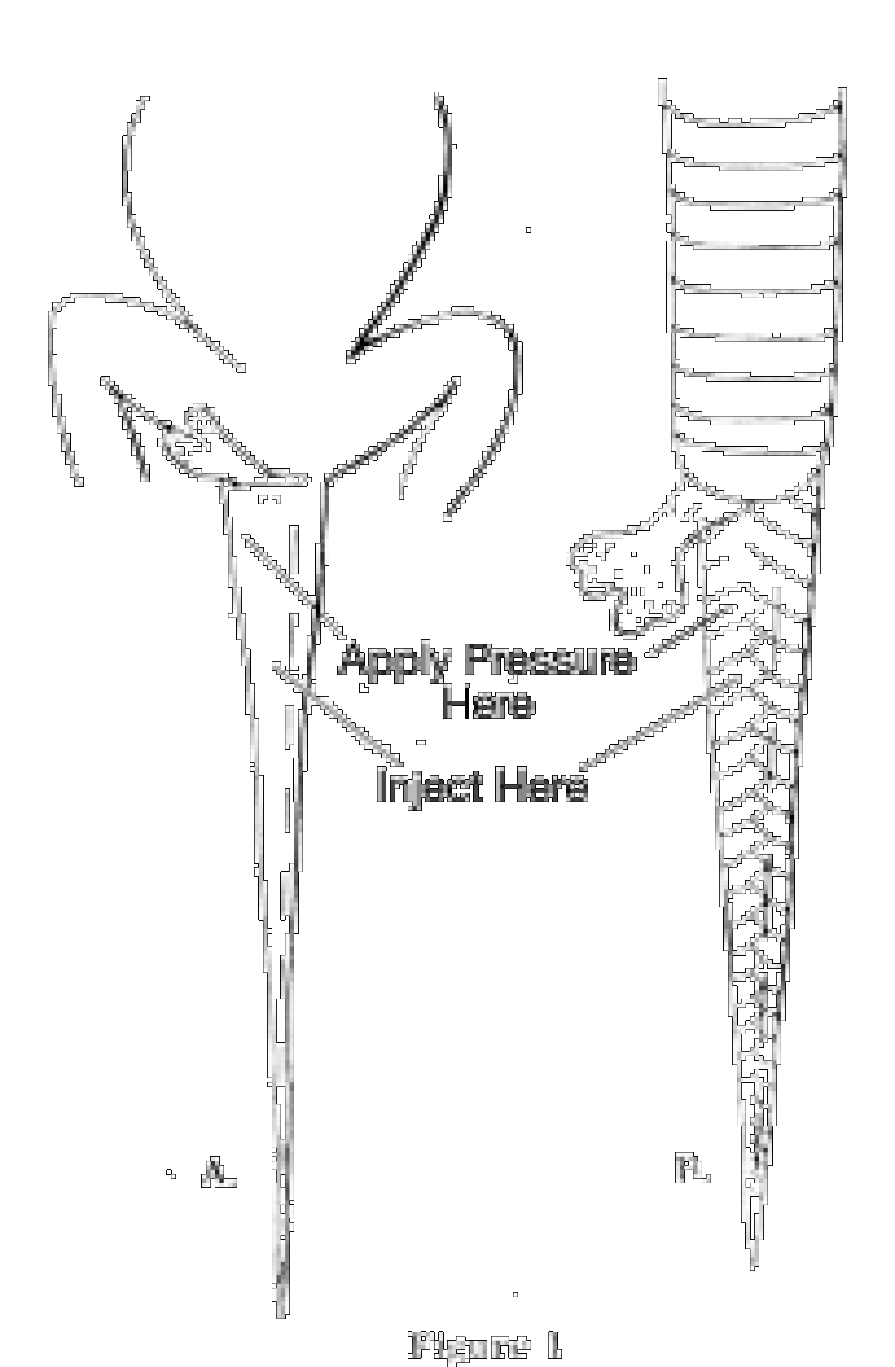 Lizards. Injection should be made through the belly directly into the body cavity. Care should be taken not to use too much, or the body will become unnaturally distended. A series of slits should be made in the under side of the tail with a sharp scalpel or razor blade. The slits should be from 1/8 to 1/4 inch long and about 1/4 inch apart, and should extend from the base of the tail to the tip. Very large lizards must be injected or slit in the thicker portions of the limbs and neck. If space does not permit preservation of very large lizards whole, they may be skinned out, except for the head. To skin a large lizard, make a cut down the belly from the neck to the base of the tail. Work the skin loose from the body, pulling the skin of the arms and legs inside out as far as the wrists and ankles. Do not attempt to skin out the head, hands, feet or tail. Sever the wrists, ankles, neck and base of the tail, and remove the carcass. The skin should then he placed directly into preservative. If possible, one hemipenis of male lizards should be everted. This can be accomplished hy injecting preservative into the base of the tail (before slitting) and at the same time applying pressure with the thumb just behind the anus (Fig. 1 A).
Lizards. Injection should be made through the belly directly into the body cavity. Care should be taken not to use too much, or the body will become unnaturally distended. A series of slits should be made in the under side of the tail with a sharp scalpel or razor blade. The slits should be from 1/8 to 1/4 inch long and about 1/4 inch apart, and should extend from the base of the tail to the tip. Very large lizards must be injected or slit in the thicker portions of the limbs and neck. If space does not permit preservation of very large lizards whole, they may be skinned out, except for the head. To skin a large lizard, make a cut down the belly from the neck to the base of the tail. Work the skin loose from the body, pulling the skin of the arms and legs inside out as far as the wrists and ankles. Do not attempt to skin out the head, hands, feet or tail. Sever the wrists, ankles, neck and base of the tail, and remove the carcass. The skin should then he placed directly into preservative. If possible, one hemipenis of male lizards should be everted. This can be accomplished hy injecting preservative into the base of the tail (before slitting) and at the same time applying pressure with the thumb just behind the anus (Fig. 1 A).
Snakes. Make a series of injections an inch or two apart through the belly into the body cavity. Begin just behind the head and continue the injections to the anus. If a syringe is not available, a series of slits must be made in the belly. For most snakes the slits should be about an inch apart and an inch long; smaller slits closer together for very small snakes. Just as in lizards, a series of slits must be made in the under side of the tail and one hemipenis everted in males (Fig. 1B). Very large snakes may be skinned out, leaving the head and tail attached. To skin a snake make a single, long cut in the belly, just to one side of the midline, beginning about an inch behind the head and continuing to about an inch in front of the anus. Do not cut through the anal plate. Work the skin loose from the body, but do not attempt to remove the skin from the head or tail. Sever the body an inch behind the head and an inch in front of the anus, and (after recording the stomach contents, number of eggs, embryos, etc.) discard the carcass. Put a strip of cloth on the inner side of the skin and roll it up, beginning at the head. Tie the roll with a piece of string and put it directly into preservative.
Alligators and Crocodiles. Small individuals may be preserved just as lizards. Larger individuals should be skinned out with the head attached, rolled up and placed directly into preservative.
Turtles. Preservative should be injected into the body cavity just in front of each of the four limbs, between the carapace and plastron. Use a long needle and continue injections until the head and · limbs are forced out of the shell. If a syringe is not available, make deep cuts into the body cavity just in front of each leg. Limbs, neck and tail should be injected or slit, as in large lizards.
 Labels and Records: Specimens for which there are no data are of little or no scientific value. It is very important that each specimen be accompanied by certain information. This information may either be printed on a label which is attached to the specimen or may be recorded in a notebook. If a notebook is used the data should be identified by a number; a tag bearing the same number should be attached to the specimen. The most important datum is the locality at which the collection was made. This should include the distance from and direction to the center or city limits (state which) of the nearest city or town which can be easily found on a map. Do not record distances to unincorporated towns or villages which are not likely to be marked on maps. If the distance along a highway is used, state which highway. In the United States, areas may be located with great accuracy by use of township, range and section maps. The name of the county and state, or of corresponding political units of foreign countries, should be included. Altitude may be of extreme importance and, if not readily ascertainable from maps of the area, it should be recorded. With the availability of inexpensive Global Positioning System (GPS) receivers, one can now easily enter precise latitude and longitude data. This precise data is desired by those researchers engaged in mapping ranges of amphibians and reptiles with GIS mapping programs. Next in importance is the date of collection. The month should be written out or a clear abbreviation used. Do not use numbers separated by dashes, such as "8-5-56". The name of the collector should be recorded. In addition to these data it is desirable to make careful descriptions of color and pattern from individuals before they are killed, since color often fades rapidly after death.
Labels and Records: Specimens for which there are no data are of little or no scientific value. It is very important that each specimen be accompanied by certain information. This information may either be printed on a label which is attached to the specimen or may be recorded in a notebook. If a notebook is used the data should be identified by a number; a tag bearing the same number should be attached to the specimen. The most important datum is the locality at which the collection was made. This should include the distance from and direction to the center or city limits (state which) of the nearest city or town which can be easily found on a map. Do not record distances to unincorporated towns or villages which are not likely to be marked on maps. If the distance along a highway is used, state which highway. In the United States, areas may be located with great accuracy by use of township, range and section maps. The name of the county and state, or of corresponding political units of foreign countries, should be included. Altitude may be of extreme importance and, if not readily ascertainable from maps of the area, it should be recorded. With the availability of inexpensive Global Positioning System (GPS) receivers, one can now easily enter precise latitude and longitude data. This precise data is desired by those researchers engaged in mapping ranges of amphibians and reptiles with GIS mapping programs. Next in importance is the date of collection. The month should be written out or a clear abbreviation used. Do not use numbers separated by dashes, such as "8-5-56". The name of the collector should be recorded. In addition to these data it is desirable to make careful descriptions of color and pattern from individuals before they are killed, since color often fades rapidly after death.
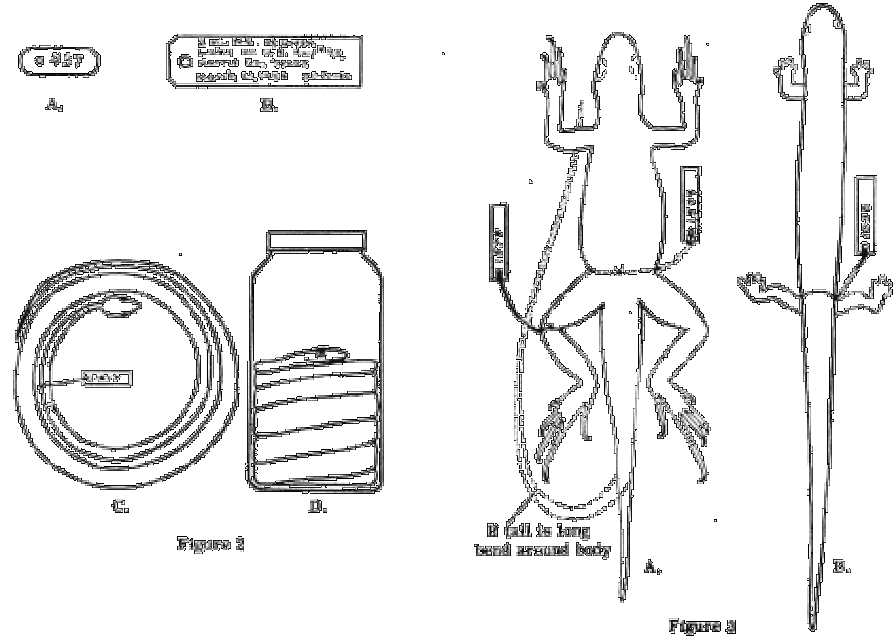
If a field notebook is used a description may be given of the habitat, climatic conditions and notes on behavior, such as the voice of calling frogs or toads, or a reference to an audiotaped call. If numbered field tags are used, a notebook should be kept in waterproof ink or soft pencil, in which each number is listed consecutively, accompanied by the above data. Use tags that are especially made for this purpose by biological supply houses, or use the best grade of "bond" or linen paper. The size and shape recommended is shown in Fig. 2 A. Tags made of laminated paper or cardboard will fall apart in liquid. Write only with a medium-soft pencil, never with ordinary ink, ball point pen or indelible pencil. "India Ink" or "Higgins Eternal Black" or Engrossing Ink may be used, but the tags should not be immersed in liquid until the ink is completely dry. The new "Pigma pens" with black alcohol-proof ink seem to be a reasonable substitute for India Ink.
 If a notebook is not used, a tag large enough to bear the locality, date and collector's name should be attached to each specimen. The size and shape of such a tag is shown in Fig. 2 B. On lizards, frogs, toads and turtles, the tag should be tied (with a square knot) immediately below the knee of the left leg. Very small frogs, lizards and all salamanders should be tied around the narrowest part of the waist. Snakes should be tagged well back from the head but in front of the thickest part of the body. (Figs. 2 C, 3 A-B, 4 A). If a large series of specimens is collected at one locality on the same day, a single tag may serve for the entire series. The tag should be tied on one of the specimens and the entire lot kept isolated from other specimens, either in a separate container or wrapped in cloth. This practice should be avoided if time permits individual tagging.
If a notebook is not used, a tag large enough to bear the locality, date and collector's name should be attached to each specimen. The size and shape of such a tag is shown in Fig. 2 B. On lizards, frogs, toads and turtles, the tag should be tied (with a square knot) immediately below the knee of the left leg. Very small frogs, lizards and all salamanders should be tied around the narrowest part of the waist. Snakes should be tagged well back from the head but in front of the thickest part of the body. (Figs. 2 C, 3 A-B, 4 A). If a large series of specimens is collected at one locality on the same day, a single tag may serve for the entire series. The tag should be tied on one of the specimens and the entire lot kept isolated from other specimens, either in a separate container or wrapped in cloth. This practice should be avoided if time permits individual tagging.
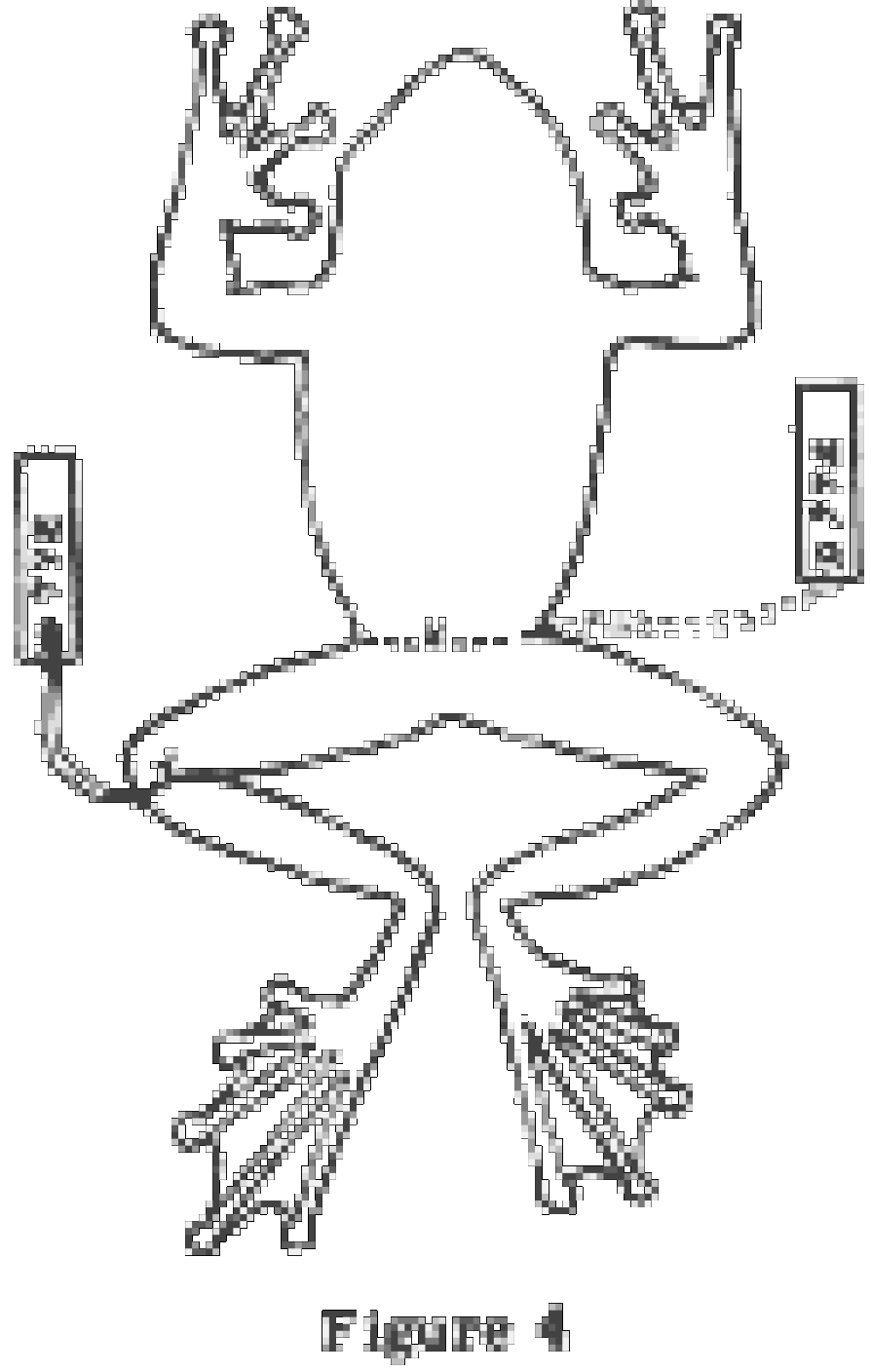 Reptiles and amphibians which have been well-fixed and allowed to harden in the positions indicated in Figs. 2 C-D, 3 A-B and 4 A have many advantages over those which have been left to harden in the posture in which they died. The extra time and care required is well worth the effort. Specimens can be more easily stored, photographed and examined and can be more accurately measured and compared if they are allowed to harden in the proper position. The equipment required is not elaborate; several shallow trays of glass, plastic or rust-proof metal, something with which to cover the trays, and paper. The bottom of the tray is covered with a single piece of paper (paper towels are good) which has been dampened with water. The specimens are placed on the paper, arranged in the desired position, and covered with another piece of damp paper. Pour enough preservative over the top to thoroughly soak the paper and allow about an eighth of an inch of liquid free in the bottom of the tray.
Reptiles and amphibians which have been well-fixed and allowed to harden in the positions indicated in Figs. 2 C-D, 3 A-B and 4 A have many advantages over those which have been left to harden in the posture in which they died. The extra time and care required is well worth the effort. Specimens can be more easily stored, photographed and examined and can be more accurately measured and compared if they are allowed to harden in the proper position. The equipment required is not elaborate; several shallow trays of glass, plastic or rust-proof metal, something with which to cover the trays, and paper. The bottom of the tray is covered with a single piece of paper (paper towels are good) which has been dampened with water. The specimens are placed on the paper, arranged in the desired position, and covered with another piece of damp paper. Pour enough preservative over the top to thoroughly soak the paper and allow about an eighth of an inch of liquid free in the bottom of the tray.
Specimens should be injected (or slit) and tagged as soon as possible after they are dead and fixing should immediately follow. Individuals may be placed close together on the tray but should not touch each other. The tray should be covered to prevent evaporation. Most amphibians will harden in a few hours, reptiles in 10 or 12 hours. Large lizards, frogs and turtles may take a little longer, but the paper should be checked at least twice a day to be sure it is not drying out.
 Snakes. Small snakes may be coiled flat in the tray if the coil does not exceed three and one half inches in its outside diameter. The head should be inside as in Fig. 2 C. Larger snakes should be coiled in a jar and covered with preservative. If the snake has been injected it may be coiled with the belly down, tail at the bottom and head on top as in Fig. 2D. If slits are used, it should be coiled with the belly up, head on the bottom and tail on top. Tall, narrow bottles should be avoided; quart and pint sizes are best. Snakes too large to coil in a gallon jar should be skinned.
Snakes. Small snakes may be coiled flat in the tray if the coil does not exceed three and one half inches in its outside diameter. The head should be inside as in Fig. 2 C. Larger snakes should be coiled in a jar and covered with preservative. If the snake has been injected it may be coiled with the belly down, tail at the bottom and head on top as in Fig. 2D. If slits are used, it should be coiled with the belly up, head on the bottom and tail on top. Tall, narrow bottles should be avoided; quart and pint sizes are best. Snakes too large to coil in a gallon jar should be skinned.
Lizards. Place the lizard belly down, with arms, legs and tail extended. If the tail is very long it may be bent around the side of the body (Fig. 3 A). Attenuate, limbless lizards may be coiled like snakes.
Turtles. Most important in fixing turtles is that the head and neck be extended and the mouth propped open with a bit of wood or cork. The limbs should also be extended if possible.
Salamanders. Belly down, arms, legs and tail extended as in Fig. 3 B. A salamander tail will often twitch back and forth long after the animal seems to be dead. Ten or fifteen minutes after they have been laid out check to be sure the tail is still straight. Large specimens, 10 or more inches in length, may be coiled like snakes.
Frogs and Toads. Place the frog belly down, arms and legs extended as in Fig. 4 A. The fingers and toes should be separated and extended, especially if they are webbed. The inner margin of the forelimb from the elbow to the tip of the fourth finger should form a straight line. The sole of the foot may be up or down, whichever seems most natural (down in treefrogs and up in most other frogs and toads).
 Storage: After the specimens have been injected or slit, tagged, and fixed, they should be put directly into preservative. If they are to be transferred later to plastic bags for temporary storage or to be shipped they should first be allowed to remain completely immersed in preservative for at least 48 hours if formalin is used, or a week if alcohol is used. The longer they are allowed to stay in preservative, the better. They should be loose and completely covered with plenty of liquid. Specimens which have been hardened in trays should also be allowed to soak in preservative for a day or two before being shipped or placed in plastic bags for storage. If space is no problem, preserved specimens are best kept in glass containers. Bail-top jars with a glass top and rubber gasket are best. Fruit jars with a metal screwtop lid may be used but should be carefully watched for rust and evaporation. Glass jars with polyethylene lids and liners are more commonly used in collections, since the lids form a tight seal and are easier to obtain than the traditional bail-top jars. Metal containers should be used only for temporary storage unless coated on the inside with paraffin, "Bakvar", or some other rustproof material.
Storage: After the specimens have been injected or slit, tagged, and fixed, they should be put directly into preservative. If they are to be transferred later to plastic bags for temporary storage or to be shipped they should first be allowed to remain completely immersed in preservative for at least 48 hours if formalin is used, or a week if alcohol is used. The longer they are allowed to stay in preservative, the better. They should be loose and completely covered with plenty of liquid. Specimens which have been hardened in trays should also be allowed to soak in preservative for a day or two before being shipped or placed in plastic bags for storage. If space is no problem, preserved specimens are best kept in glass containers. Bail-top jars with a glass top and rubber gasket are best. Fruit jars with a metal screwtop lid may be used but should be carefully watched for rust and evaporation. Glass jars with polyethylene lids and liners are more commonly used in collections, since the lids form a tight seal and are easier to obtain than the traditional bail-top jars. Metal containers should be used only for temporary storage unless coated on the inside with paraffin, "Bakvar", or some other rustproof material.
Specimens should be loosely packed and completely covered with liquid. Containers must be periodically checked for evaporation and refilled if necessary. At the first sign of decomposition the affected specimen should be removed and thrown away, or deep cuts made into the body cavity and placed alone in a large container with plenty of fresh preservative. A green spot on the abdomen of a snake or lizard indicates a rotten gall bladder which should be cut out. Any specimen that floats in the preservative contains air or other gases and is not properly preserved. It should be squeezed or slit to permit the gases to escape and the preservative to enter.
When traveling in the field, it is often impossible to carry along a large number of glass jars for storage. If specimens are well preserved and have been immersed in preservative for several days, it is safe to store them in plastic bags for a period of several months. Plastic bags are inexpensive and sold in various sizes. Whirl-Pak Bags are ideal for field collections, as they are made of heavy-duty polyethylene and are leakproof when properly closed. Specimens should be wrapped loosely in cheesecloth. An easy method is to cut a strip of cloth, lay it flat on a table and arrange the specimens in a row on the cloth with an inch or two between them.
The strip should then be rolled loosely with the specimens. Put the roll in a plastic bag and add enough preservative to soak the cloth and have a little free liquid in the bottom. Twist the open end of the bag and wrap it tightly with a rubber band. Many museums use heat-sealed rolls of wide polytehylene tubing, which allows bags in any size with heat-sealed seams. This is an efficient way to handle large numbers of specimens for shipping. A number of such bags may be stored in a metal can, but care should be taken to put bags with large, heavy specimens on the bottom.
Shipping: If your collection has been stored in plastic bags, simply fill up the container in which the bags are stored with wads of cloth or cotton so the bags will not knock about in transit, fasten the lid down tightly, and put the container in a wooden or heavy cardboard box for shipment. If plastic bags are not available, wrap the specimens loosely in cheesecloth and pack them carefully in a water-tight metal or plastic (polyethylene) container. If the bundles do not fill up the container, fill it up with wads of cloth or cotton. Never use paper, leaves or wood chips. Pour in enough preservative to soak the cloth. No free liquid is necessary if the specimens are well preserved. Small bottles with tight screw caps containing tadpoles, salamander larvae or other delicate specimens should be wrapped with cloth to prevent breakage, and placed among the bundles of wrapped specimens.

